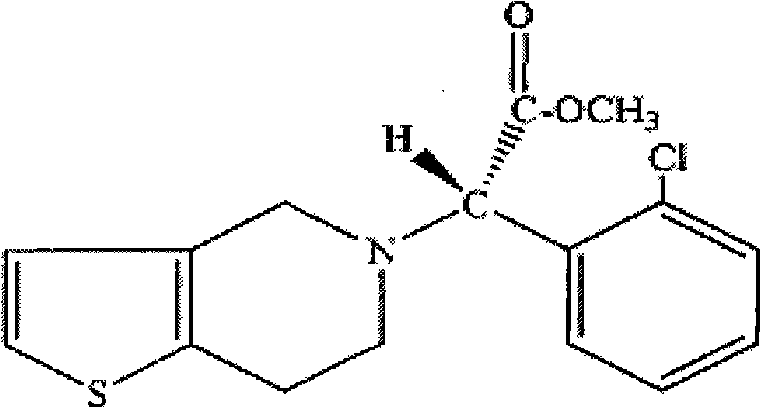
Compound sustained-release preparation of aspirin and clopidogrel or pharmaceutically acceptable salt thereof
A technology of aspirin and clopidogrel, which is applied in pharmaceutical formulations, medical preparations containing active ingredients, drug delivery, etc., to achieve the effects of avoiding peak and valley phenomena, reducing adverse reactions, and reducing drug concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0044] The compound matrix sustained-release tablet of example 1 aspirin and clopidogrel bisulfate
[0045] Aspirin 75mg
[0046] Clopidogrel Bisulfate 97.875mg
[0047] Hypromellose 150mg
[0048] Lactose 50mg
[0049] Magnesium stearate 3~6mg
[0050] Proper amount of hypromellose ethanol solution
[0051] Preparation process: aspirin, clopidogrel bisulfate, and hydroxypropylmethylcellulose, a matrix slow-release material, are pulverized and sieved. Weigh the prescribed amount of aspirin, clopidogrel bisulfate, mix them with hypromellose and lactose respectively, then add the ethanol solution of hypromellose respectively, mix to make soft material, and granulate with 24-30 mesh nylon sieve , ventilated and dried at a temperature of 40°C to 50°C for 1.5 to 2.5 hours, sized, then mixed with aspirin sustained-release granules and clopidogrel bisulfate sustained-release granules, added magnesium stearate, and compressed into tablets to obtain. ...
example 2
[0053] Example 2 Compound aspirin / clopidogrel double-layer matrix sustained-release tablet
[0054] Aspirin extended-release portion:
[0055] Aspirin 150mg
[0056] HPMC K4M 110mg
[0057] Citric acid 7.5mg
[0059] Appropriate amount of PVP ethanol solution
[0060] Clopidogrel extended-release fraction:
[0061] Clopidogrel 75mg
[0062] Pregelatinized starch 40mg
[0063] HPMC K15M 70mg
[0064] Magnesium stearate 6~8mg
[0065] Appropriate amount of PVP ethanol solution
[0066] Preparation process: aspirin slow-release part: weigh the prescribed amount of aspirin, HPMC K4M , citric acid, and set aside; clopidogrel slow-release part: the prescribed amount of clopidogrel bisulfate, pregelatinized starch, HPMC K15M Mix evenly and set aside; add PVP ethanol solution to the aspirin slow-release part and clopidogrel slow-release part respectively, mix to make a soft mate...
example 3
[0068] Example 3 Compound aspirin / clopidogrel bisulfate double-layer matrix sustained-release tablet
[0069] Aspirin 150mg
[0070] HPMC K15M 100mg
[0071] Citric acid 5mg
[0073] Clopidogrel Bisulfate 97.875mg
[0074] Microcrystalline Cellulose 20mg
[0075] Pregelatinized starch 60mg
[0076] Crospovidone (PVPP) 15mg
[0077] Zinc stearate 3~6mg
[0078] Preparation process: aspirin, HPMC K15M , citric acid, and talcum powder were mixed uniformly by equal increment method, and set aside; the prescribed amount of clopidogrel bisulfate, pregelatinized starch, microcrystalline cellulose, PVPP zinc stearate were uniformly mixed by equal increment method , ready for use; first pre-compress the aspirin slow-release part, then pour into the clopidogrel bisulfate immediate-release part, press into tablets, and obtain.
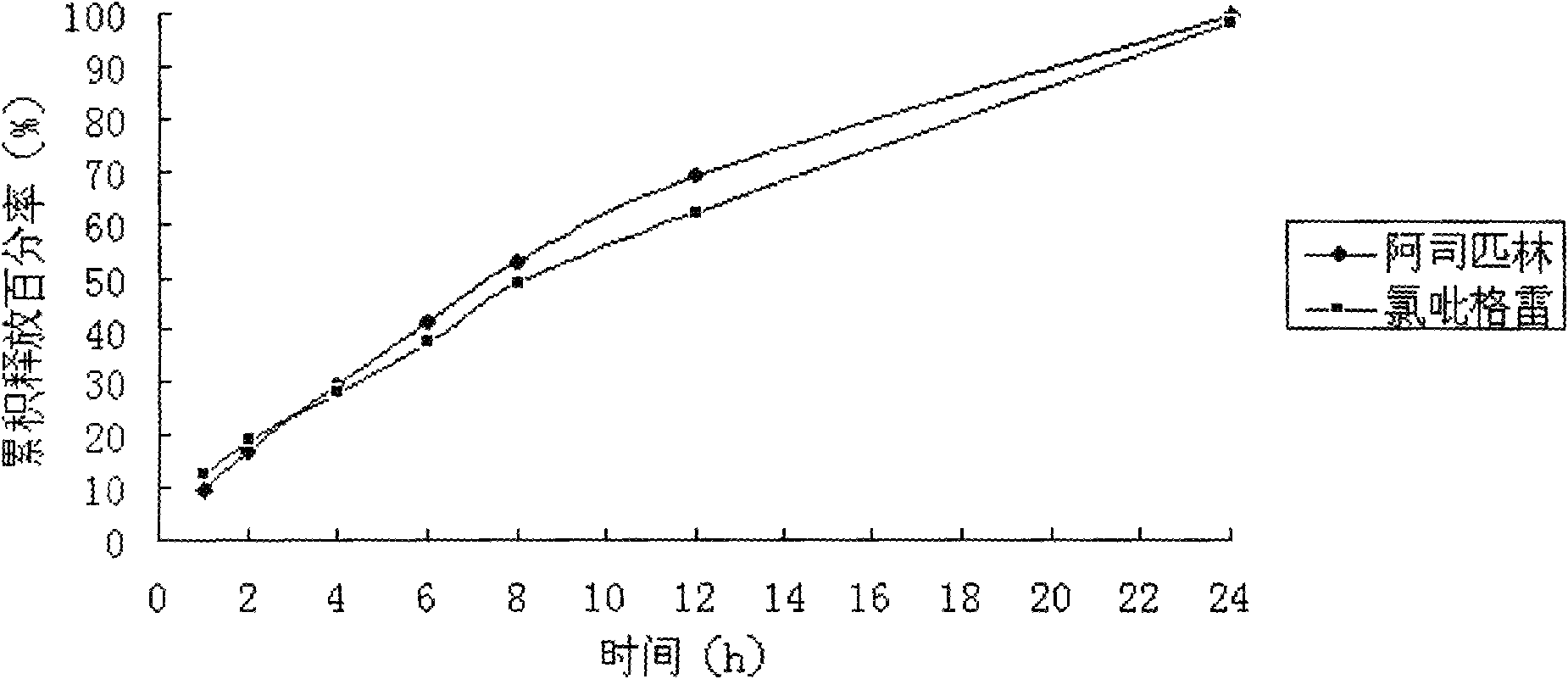
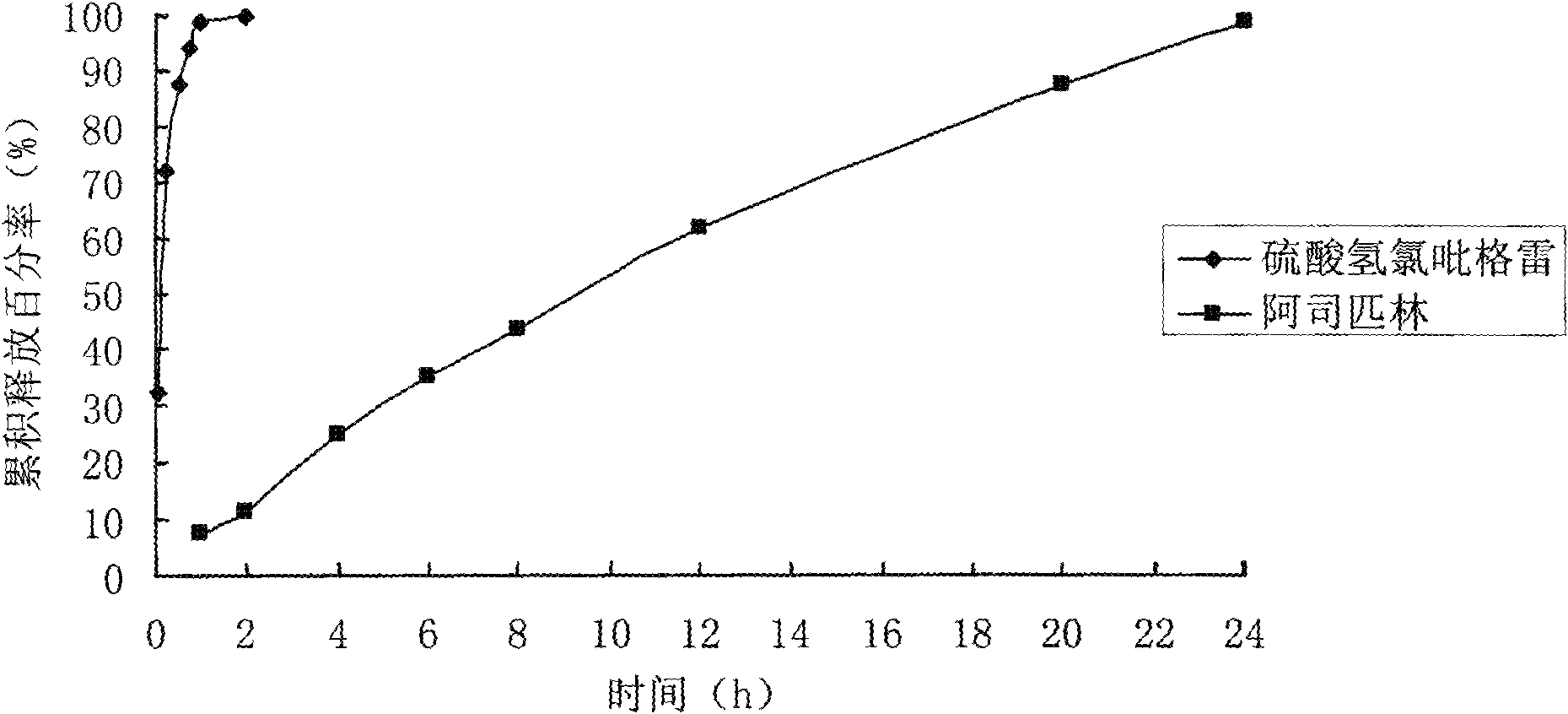
[0079] Determination of the release rate of the sustained-release tablet:...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com