Artificial synthesis method of 1,4,5,6-tetrahydro-2-methyl-4-pyrimidine carboxylic acid
A pyrimidine carboxylic acid, artificial synthesis technology, applied in 1 field, to achieve the effect of simple synthesis process steps and little environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
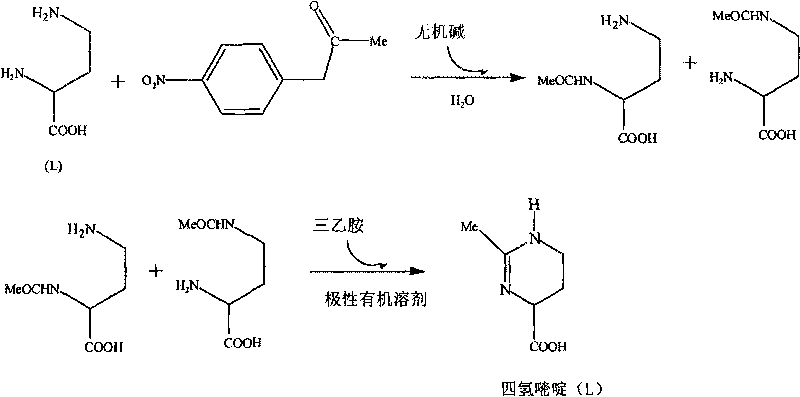
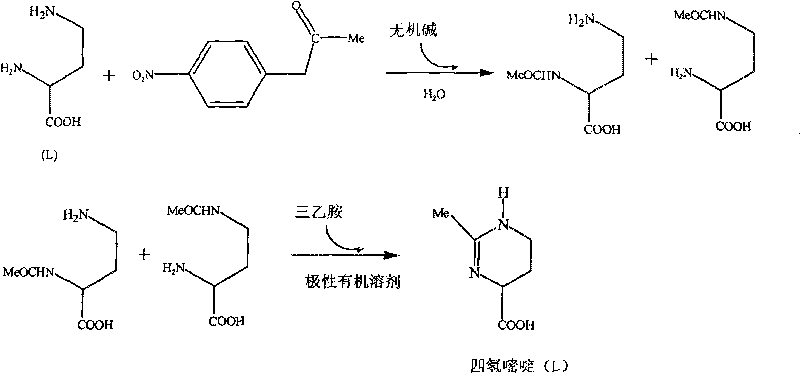
[0027] Example 1 Synthetic preparation of 1,4,5,6-tetrahydro-2-methyl-4-pyrimidinecarboxylic acid, the steps are as follows:
[0028] (1) Mix 4-nitrophenol acetate (3.62g, 20mmol) with NaOH (1.68g, 40mmol), add to 200ml L-2,4-diaminobutyric acid aqueous solution (0.1mmol / ml), at 25°C Stirring at 80rpm for 14 hours, after the completion of the reaction, with CH 2 Cl 2 Extraction was carried out, the extract was dried with anhydrous sodium sulfate, and CH was distilled off under reduced pressure. 2 Cl 2 and the remaining 4-nitrophenol acetate, and vacuum-dried at 50°C for 4 hours to obtain a mixture of 2-acetamido-4-aminobutyric acid and 2-amino-4-acetamidobutyric acid.
[0029] (2) Dissolve the mixture of 2-acetamido-4-aminobutyric acid and 2-amino-4-acetamidobutyric acid in 80ml of n-butanol, add 2g of triethylamine, heat and reflux at 80°C for 48 hours, and depressurize The organic solvent was evaporated, and column chromatography (200-300 mesh silica gel) was performed w...
Embodiment 2
[0035] Example 2 Artificially synthesized 1,4,5,6-tetrahydro-2-methyl-4-pyrimidinecarboxylic acid, the steps are as follows:
[0036] (1) Mix 4-nitrophenol acetate (5.43g, 30mmol) with KOH (2.25g, 40mmol), add to 200ml L-2,4-diaminobutyric acid aqueous solution (0.1mmol / ml), at 25°C Stirring at 80rpm for 12 hours, after the completion of the reaction, with CH 2 Cl 2 Extraction was carried out, the extract was dried with anhydrous sodium sulfate, and CH was distilled off under reduced pressure. 2 Cl 2 and the remaining 4-nitrophenol acetate, and vacuum-dried at 50°C for 4 hours to obtain a mixture of 2-acetamido-4-aminobutyric acid and 2-amino-4-acetamidobutyric acid.
[0037] (2) Dissolve the mixture of 2-acetamido-4-aminobutyric acid and 2-amino-4-acetamidobutyric acid in 100ml of ethanol, add 2g of triethylamine, heat and reflux at 120°C for 48 hours, evaporate under reduced pressure Organic solvent, column chromatography (200-300 mesh silica gel) with n-butanol / acetic a...
Embodiment 3
[0043] Example 3 Artificially synthesized 1,4,5,6-tetrahydro-2-methyl-4-pyrimidinecarboxylic acid, the steps are as follows:
[0044] (1) Mix 4-nitrophenol acetate (5.43g, 30mmol) with NaOH (1.68g, 40mmol), add to 200ml L-2,4-diaminobutyric acid aqueous solution (0.1mmol / ml), at 30°C Stirring at 80rpm for 14 hours, after completion of the reaction, with CH 2 Cl 2 Extraction was carried out, the extract was dried with anhydrous sodium sulfate, and CH was distilled off under reduced pressure. 2 Cl 2 and the remaining 4-nitrophenol acetate, and vacuum-dried at 50°C for 4 hours to obtain a mixture of 2-acetamido-4-aminobutyric acid and 2-amino-4-acetamidobutyric acid.
[0045] (2) Dissolve the mixture of 2-acetamido-4-aminobutyric acid and 2-amino-4-acetamidobutyric acid in 80ml of n-butanol, add 2g of triethylamine, heat and reflux at 120°C for 36 hours, and depressurize The organic solvent was evaporated, and column chromatography (200-300 mesh silica gel) was performed with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com