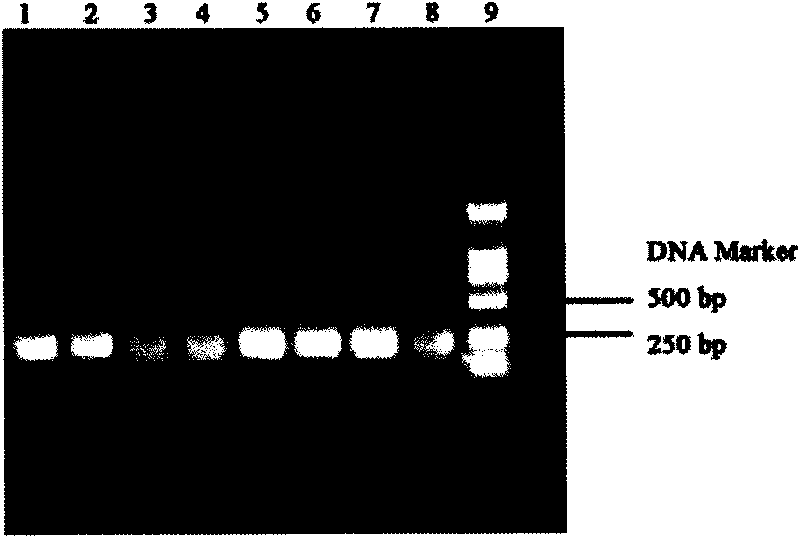
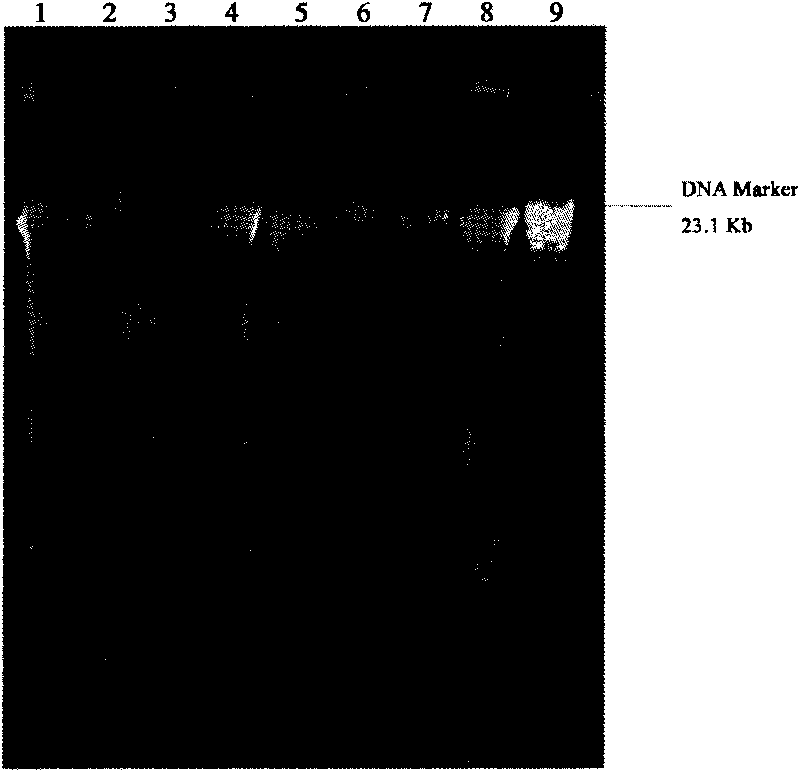
DNA extraction method suitable for structural analysis of microbial community in sediment
A technology for microbial community and structure analysis, applied in the field of DNA extraction for microbial community structure analysis in sediments, can solve problems such as high cost, difficult PCR amplification, and inability to effectively remove, and achieves the effect of reducing experimental costs and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Weigh 0.5g sediment sample (wet weight) into a 5mL sterilized centrifuge tube, add 3mL TENPbuffer, vortex for 8min, centrifuge at 10000rpm for 5min, discard the supernatant, add 3mL TENPbuffer to the soil particle sedimentation, press Wash twice more by the above method, and finally add 3mL of PBS buffer. The pretreated sediment samples were placed at -80°C and 65°C for three times to freeze and thaw repeatedly, and lysozyme (final concentration was 1 mg / mL) was added to the samples, and placed in a water bath at 37°C for 1 h. Add proteinase K (final concentration: 0.2mg / mL) and sodium dodecylsulfonate (SDS) (final concentration: 1%, w / v) to the sample, and place in a water bath at 55°C for 1.5h (light every 10-15min) Gently invert the centrifuge tube several times to mix). Centrifuge at 10,000 rpm for 5 min at room temperature, take out 500 μL of the supernatant and place it in a new centrifuge tube, add an equal volume of phenol:chloroform:isoamyl alcohol mixture (vo...
Embodiment 2
[0031] The DNA extracted in Example 1 is used as the template of the polymerase chain reaction (PCR), using the PCR amplification instrument of the U.S. Bio-Rad company, adopting the universal primer pair GC-F341 and R518 of the 16S rRNA gene V3 region of bacteria For amplification, the specific sequence of the primers is: F341 (5'-CCTACGGGAGGCAGCAG-3'); R518 (5'-ATTACCGCGGCTGCTGG-3'), GC hairpin structure (5'-CGCCCGCCGCGCGCG GCGGGCGGGGCGGGGGCACGGGGGC-3'). (Provided by Shanghai Sangon Bioengineering Technology Service Co., Ltd.)
[0032] The 50 μL PCR reaction system includes: DNA template 50ng, forward and reverse primers 20pmol each, 200μM dNTP, 10×PCR buffer (without MgCl 2 )5μL, 1.5mM MgCl 2 , 1U of ExTaq DNA polymerase and an appropriate amount of double distilled water to make up 50 μL.
[0033] The PCR reaction adopts the landing PCR strategy, namely: 94 °C pre-denaturation for 5 min, the first 20 cycles are 94 °C for 1 min, 65-55 °C for 1 min and 72 °C for 3 min (the...
Embodiment 3
[0035] Weigh 0.5g sediment sample (wet weight), add 5mL DNA extraction buffer (100mM Tris-HCl, pH8.0, 100mM EDTA, pH8.0, 100mM Na 3 PO 4 Buffer, pH8.0, 1.5M NaCl, 1% CTAB, w / v) and proteinase K (final concentration: 200 μg / mL), mix well, shake horizontally (37°C, 225rpm) in a shaker for about 30min, Add 1mL TE (10mM Tris-HCl, 1mM EDTA, pH8.0) and lysozyme (final concentration: 1mg / mL), continue to shake for 30min, then add 0.5mL 20% (W / V) SDS, put in a 65℃ water bath After 30 minutes, add 2% PVPP (w / v), centrifuge at 10,000 rpm for 10 minutes, transfer the supernatant to a new centrifuge tube, and repeat the extraction of the precipitate twice. The steps are: add DNA extraction buffer and 20% of SDS (w / v), water bath at 65°C for 10min, centrifuge at 10000rpm for 10min, collect the supernatant extracted three times in the same centrifuge tube, add RNaseA (final concentration is 200μg / mL), act at 37°C for 15min, add Equal volume of phenol: chloroform: isoamyl alcohol (25:24:1)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com