Application of alkannin derivant
A derivative, shikonin technology, applied in the application field of shikonin derivatives, can solve problems such as modification and optimization, not found, and unclear anti-tumor effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The extracts were combined, concentrated under reduced pressure until the solution turned purple, and about 250 g of crude product of purple-brown oil was obtained.
[0031] After removing the insoluble matter by filtration, add concentrated HCl to acidify the solution until the solution changes from blue to purple, at this time a large amount of precipitates are formed, and stand overnight.
[0032] Dissolve the crude product in chloroform, add 17 grams of 200-300 mesh silica gel per 50 ml, stir evenly and put it on the column. The eluent was respectively petroleum ether (60-90°C)-acetone and petroleum ether (1:1)-acetone-chloroform (1:1), and the eluents were collected in sequence.
[0033] Concentrate the eluate until the precipitate is separated out, and then recrystallize (recrystallization method refers to the Chinese patent No. 02114973.9), purify the shikonian compound, and the product is determined to be acetylshikonin by MNR, MSUV and elemental analysis data, ...
Embodiment 2
[0043] Select lung cancer cells NIH-H460 (ATCC, HTB-177) and cervical cancer cells HeLa (ATCC, CCL-2), use RPM1640 medium (purchased from Hyclone), and culture them in 24-well plates at 37°C and 5% carbon dioxide incubator In the tissue culture plate, the medium was changed after 24 hours, and the drug (without serum) was added after 16 hours of starvation. Shikonin derivatives were dissolved in DMSO (DMSO final concentration<0.1%), and the control group was treated with the same concentration of DMSO, and the specific treatment was as follows:
[0044] (1) Control group (DMSO)
[0045] (2) SK03 group
[0046] (3) SK06 group
[0047] (4) SK07 group
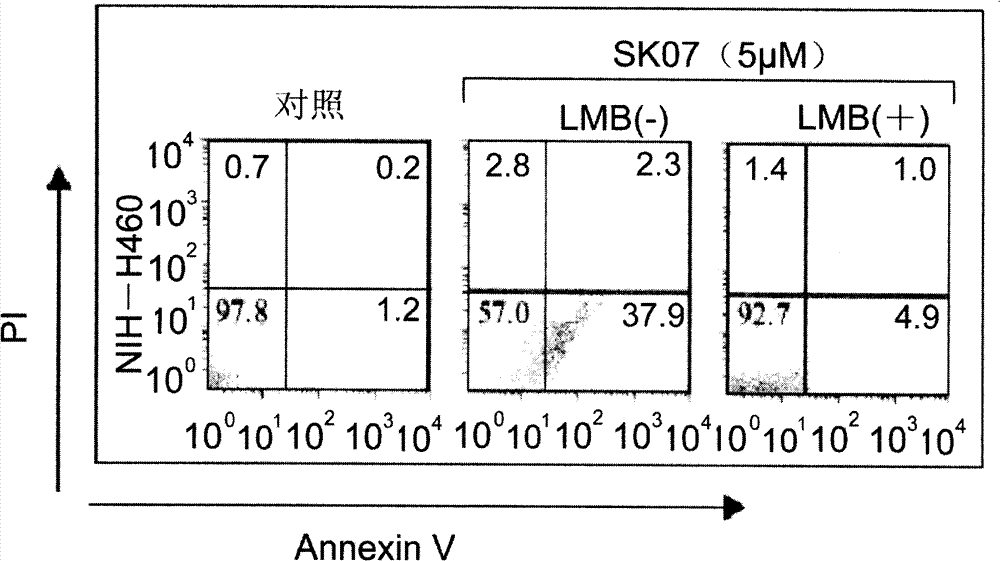
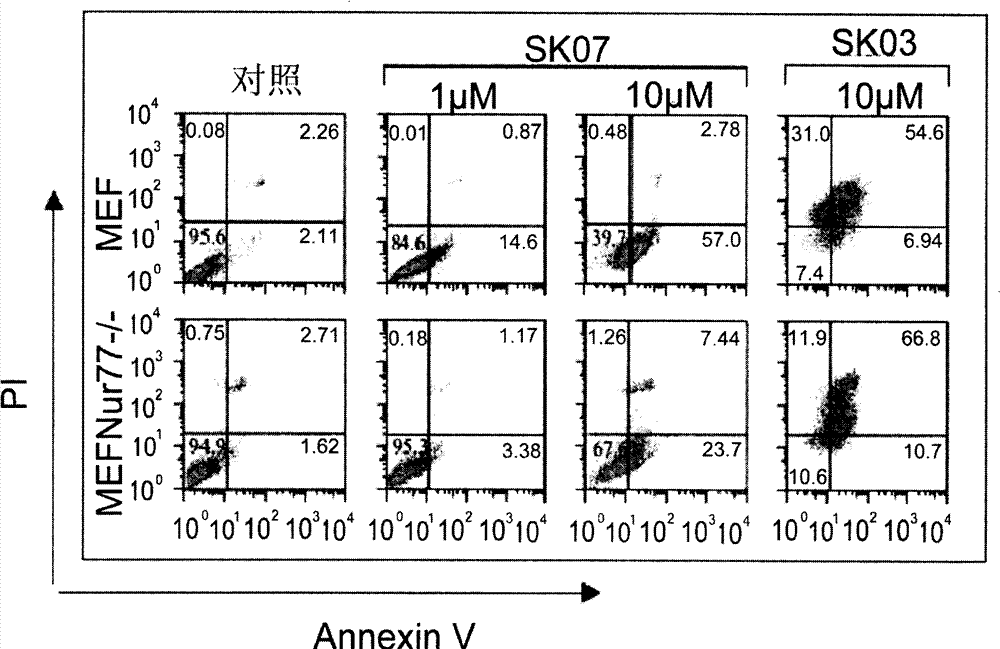
[0048] The concentrations of SK03, SK06, and SK07 were all 5 or 10 μM, and the treatment time was 12 or 24 hours. NIH-H460 cells and HeLa cells without any treatment were used as controls. In order to confirm the time-effect and dose-effect relationship, NIH-H460 cells were treated with SK07 at a concentration of 1, 5 or 10 μM...
Embodiment 3
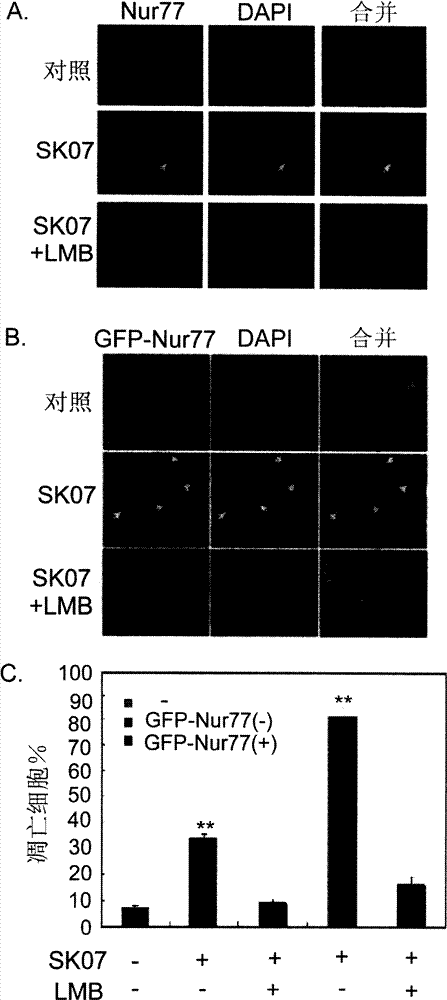
[0071] Select lung cancer cell NIH-H460 (ATCC, HTB-177), use RPM1640 medium (purchased from Hyclone), and cultivate it in a 24-well tissue culture plate at 37 ° C and 5% carbon dioxide incubator, change the medium and carry out after 24 hours Dosing (without serum) treatment. Shikonin derivatives were dissolved in DMSO (DMSO final concentration <0.1%), and the control group was treated with the same concentration of DMSO, and the specific treatment was as follows:
[0072] (1) Control group (DMSO)
[0073] (2) SK03 group
[0074] (3) SK06 group
[0075] (4) SK07 group
[0076]The total volume of each well solution in the reaction plate is 1 ml, and the concentrations of SK03, SK06 and SK07 are all 5 μM.
[0077] After 24 hours of treatment, the cells were digested with 0.5% trypsin, washed with 0.1M PBS, fixed with 4% paraformaldehyde, stained with 1 μg / ml DAPI, and observed the morphology of the nucleus with a fluorescence microscope (Carl Zeiss). The results are as follo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com