Novel benzamide derivatives as modulators of the follicle stimulating hormone
A technology of solvates and compounds, applied in the field of new benzamide derivatives as follicle-stimulating hormone regulators, can solve the problems of inducing secondary effects, non-oral activity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
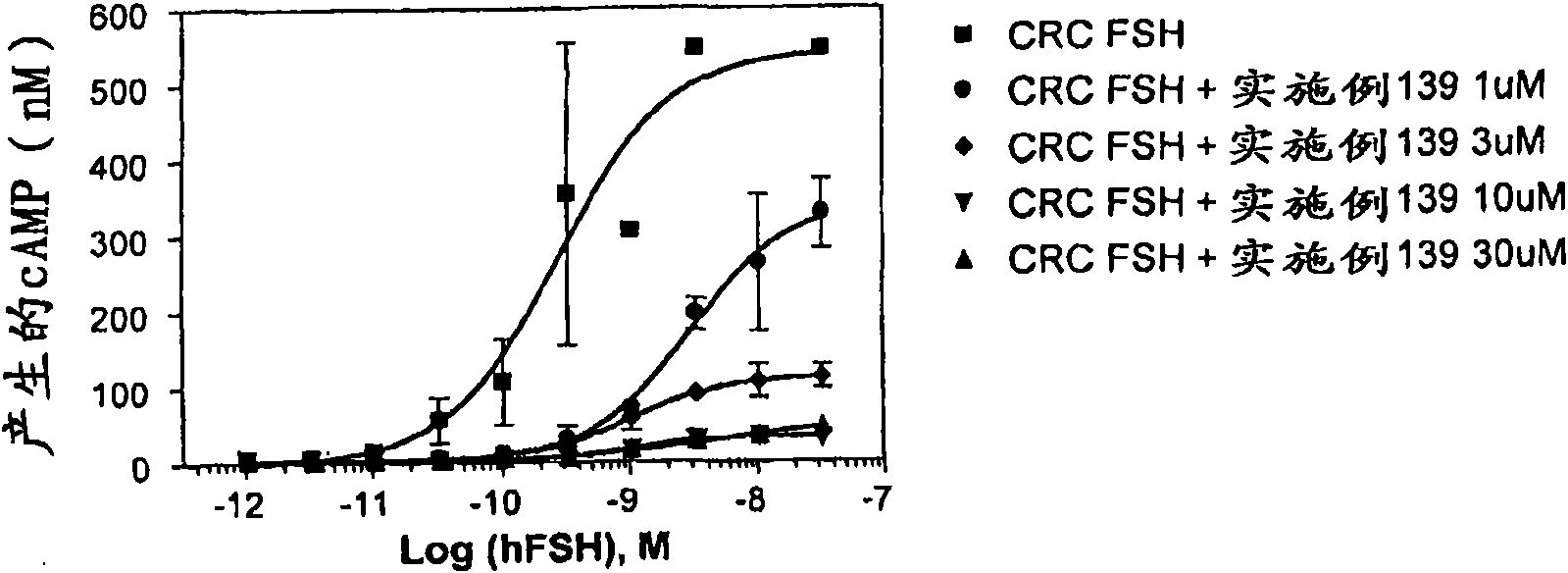
Image
Examples
Embodiment 1
[0791] N-[4-(cyano-dimethyl-methyl)-phenyl]-3,4-dimethoxy-benzamide.
[0792] 1(A) 2-Methyl-2-(4-nitro-phenyl)-propionitrile.
[0793] to at 0°C and N 2 To a solution of (4-nitro-phenyl)-acetonitrile (5.00 g; 30.9 mmol) in dry DMF (30 mL) cooled under atmosphere was added gradually NaH (60% dispersion in mineral oil; 1.23 g; 30.9 mmol) and the mixture was stirred at 0 °C for 15 min. Iodomethane (1.92 mL; 30.9 mmol) was then added and the mixture was stirred at room temperature for 1.5 hours. The reaction was recooled at 0° C. and further NaH (60% dispersion in mineral oil; 1.23 g; 30.9 mmol) was added gradually. After stirring at 0 °C for 15 min, iodomethane (1.92 mL; 30.9 mmol) was added and the reaction was stirred at room temperature for 16 hours. The solvent was removed in vacuo and the residue was taken up with EtOAc, washed with brine, Na 2 SO 4 Dry with, filter and concentrate in vacuo. The crude product was purified by chromatography [SiO 2 , Petroleum ether / ...
Embodiment 2
[0804] N-[4-(1-cyano-cyclopropyl)-phenyl]-3,4-dimethoxy-benzamide.
[0805] 2(A) 1-(4-Nitro-phenyl)-cyclopropanenitrile.
[0806] KNO was cooled in an ice-acetone bath 3 (1.10g; 10.8mmol) in concentrated H 2 SO 4 (9 mL) was gradually added to 1-phenyl-cyclopropanenitrile (1.50 g; 10.8 mmol) in concentrated H 2 SO 4 (9 mL). The reaction was stirred at ambient temperature for 1.5 hours and then poured onto ice. The precipitate was filtered, dissolved in EtOAc and washed with water and then brine. use Na 2 SO 4 The organic phase was dried, filtered and evaporated in vacuo to give Title compound , as a yellow solid (1.30 g). This compound was used in the next step without any additional purification.
[0807] 2(B) 1-(4-Amino-phenyl)-cyclopropanenitrile.
[0808] According to Example 1(B) starting material was 1-(4-nitro-phenyl)-cyclopropanenitrile (1.30 g; 6.91 mmol) as prepared in Example 2(A), and using 10% Pd / C (20 mg) was prepared in MeOH (30 mL). The catal...
Embodiment 3
[0815] 2,3-Dihydro-benzo[1,4]dioxin-6-carboxylic acid [4-(1-cyano-cyclopentyl)-phenyl]-amide.
[0816] 3(A) 1-(4-Nitro-phenyl)-cyclopentanenitrile.
[0817] (4-Nitro-phenyl)-acetonitrile (6.00 g; 37.0 mmol) and 1,4-dibromo-butane (4.42 ml; 37.0 mmol) in DMSO / Et 2 A solution in O (20 mL / 20 mL) was added dropwise to a suspension of NaH (60% dispersion in mineral oil; 1.80 g; 81.4 mmol) in DMSO (20 mL), keeping the temperature below 30 °C. After stirring at room temperature for 1 day, the 2 O (5 mL) terminated the reaction. The mixture was concentrated in vacuo and the resulting aqueous solution was treated with 2N hydrochloric acid and washed with Et 2 O extracted three times. The organic layer was collected, washed with brine, washed with Na 2 SO 4Dry, filter and concentrate to dryness in vacuo. The crude compound was purified by chromatography [SiO 2 , Petroleum ether / EtOAc(9 / 1-8 / 2)] to get Title compound , as an orange solid (3.94 g; 50% yield).
[0818] LCMS (RT...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com