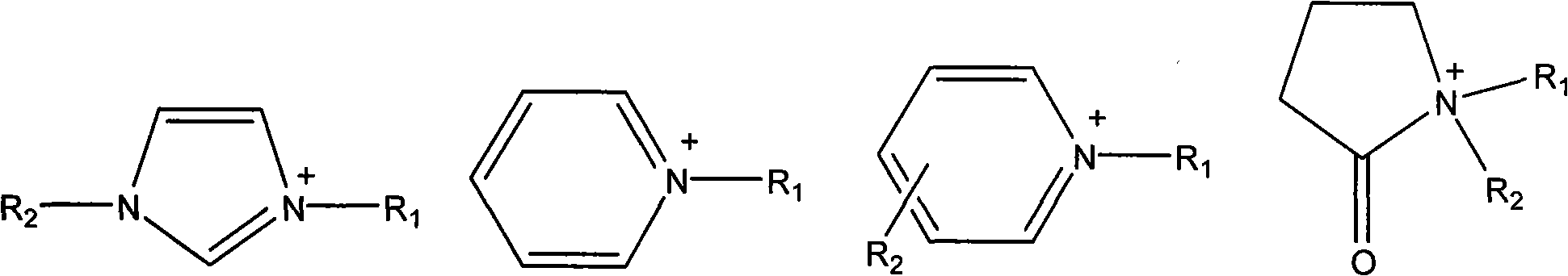
Ionic liquid containing cinnamyl functional groups and preparation method thereof
An ionic liquid and functional-based technology, which is applied in the field of ionic liquid preparation, can solve the problems of unsuitability for large-scale industrial production applications, strong corrosion of equipment, complex synthesis process, etc., and achieve post-processing green environmental protection, simple preparation process, Post-processing simple effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0043] Synthesis of 1-cinnamyl-3-methylimidazolium chloride salt: mix 8.2g (0.1mol) N-methylimidazole with 15.2g (0.1mol) cinnamyl chloride, stir at room temperature for 3 hours to obtain a light yellow viscous liquid , add 5 mL of acetonitrile or chloroform, continue the reaction for 1 hour, remove the solvent with a rotary evaporator, wash with ethyl acetate (2 × 20 mL), and obtain a light yellow viscous liquid, which is the target product, with a yield of 94%. 1 HNMR (300Hz, D 2 O), δ: 8.55 (s, 1H, NCH=N), 7.37 (d, 2H, NCH=CHN), 7.31-7.25 (m, 5H, C 6 h 5 ), 6.67 (d, 1H, C 6 h 5 CH), 6.27 (m, 1H, CHCH 2 ), 4.77 (d, 2H, CHCH 2 ), 3.73 (s, 3H, NCH 3 ).
example 2
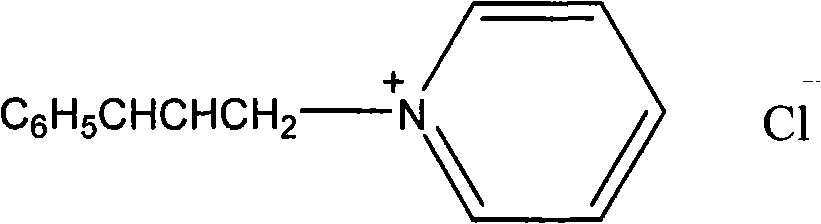
[0045] Synthesis of N-cinnamyl pyridinium chloride salt: 7.9g (0.1mol) pyridine was mixed with 15.2g (0.1mol) cinnamyl chloride, stirred at room temperature for 2 hours, washed with ethyl acetate (2×20mL), and brown viscous was obtained The liquid is the target product, and the yield is 93%. 1 HNMR (300Hz, CDCl 3 )δ: 9.7, 8.41, 8.06 (d, t, t, 5H, C 5 h 5 N), 7.37-7.23 (m, 5H, C 6 h 5 ), 7.14(d, 1H, C 6 h 5 CH), 6.52 (m, 1H, CHCH 2 ), 5.90 (d, 2H, CHCH 2 ).
example 3
[0047] Synthesis of N-methyl-N-cinnamylpyrrolidone chloride salt: 9.9g (0.1mol) N-methylpyrrolidone and 15.2g (0.1mol) cinnamyl chloride were mixed, set the temperature at 30°C, stirred for 2 hours, and dissolved in ethyl acetate The ester was washed (2×20 mL) to obtain a light yellow viscous liquid, which was recrystallized with a mixed solvent of ethanol and ether (volume ratio 2:1) to obtain the target product with a yield of 93%. 1 HNMR (300Hz, CDCl 3 )δ: 7.72-7.38 (m, 5H, C 6 h 5 ), 7.31(d, 1H, C 6 h 5 CH), 7.01(d, 2H, CHCH 2 N), 6.41 (m, 1H, CHCH 2 ), 5.18 (d, 2H, NCH 2 CH 2 ), 4.05 (s, 3H, NCH 3 ), 4.00 (t, 2H, COCH 2 ), 3.71 (m, 2H, CH 2 -CH 2 -CH 2 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com