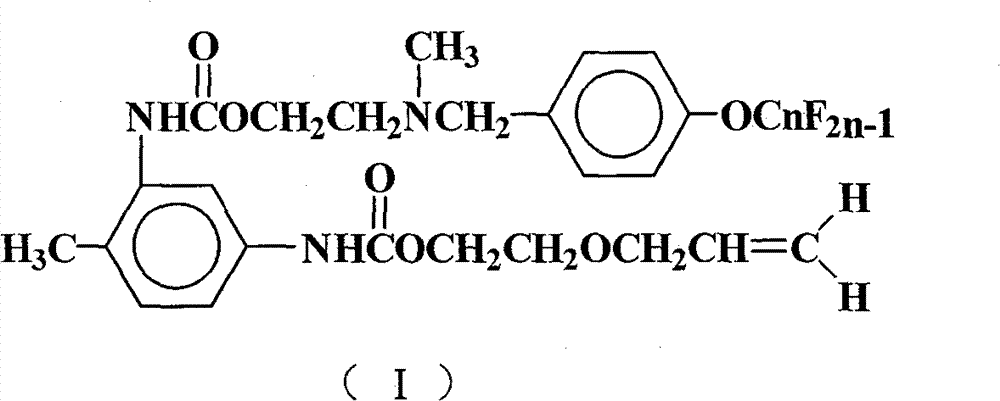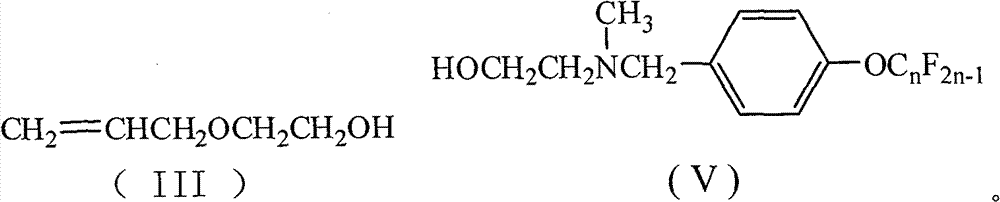Hexafluoropropylene-oligomer-containing polymer monomer as well as synthesizing method and application thereof
A technology for polymerizing monomers and hexafluoropropylene, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., to achieve the effect of convenient operation and simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
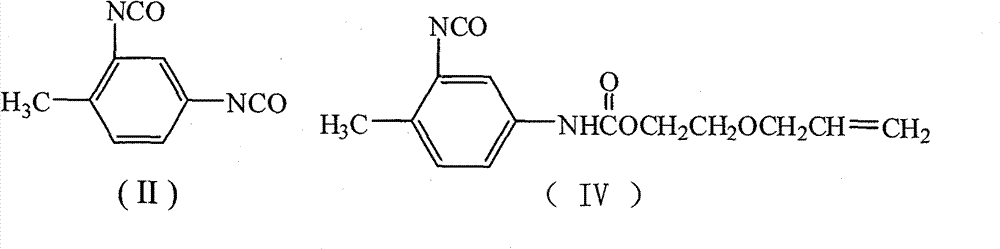
[0027] Add 15 mL of 1,2-dichloroethane, 1.74 g (10 mmol) of toluene-2,4-diisocyanate, 0.19 g (0.8 mmol) of tetraethyltin, and 0.05 g of p-hydroxyanisole into a 50 mL four-necked flask. Polymerizing agent, stirred, heated up to 75°C, added dropwise 1.02g (10mmol) of ethylene glycol monoallyl ether, after the dropwise addition, TLC detection, followed the reaction process, kept the temperature and stirred for 4h. 6.11 g (10 mmol) of N-methyl-N-hydroxyethyl-4-perfluorononenyloxybenzylamine was added dropwise. After the dropwise addition was completed, TLC was detected to track the reaction progress, and the reaction was carried out while maintaining the temperature and stirring for 5 hours. Reaction is completed, and solvent extraction is separated by silica gel column chromatography (V 二氯甲烷 :V 乙酸乙酯 :V 环己烷 =5:4:1), the light yellow paste I was obtained after removing the solvent 1 (n=9), the polymerized monomer containing hexafluoropropylene oligomer represented by formula (I)...
Embodiment 2
[0032] Carry out the reaction according to the method of Example 1, but N-methyl-N-hydroxyethyl-4-perfluorononenyloxybenzylamine is changed to N-methyl-N-hydroxyethyl-4-perfluorohexene Base oxybenzylamine 4.61g (10mmol), other operation is as embodiment 1, obtains the light yellow paste of polymerized monomer containing hexafluoropropylene oligomer shown in formula (I), I 2 (n=6), yield 66.3%, purity 99.3%.
[0033] 19 F NMRδ(CDCl 3 ): -129.4(t, 3F), -127.4(s, 3F), -121.6(m, 2F), -83.3(s, 2F), -78.1(t, 1F).
Embodiment 3
[0035] Add 15mL of N,N-dimethylformamide (DMF), 1.74g (10mmol) of 2,4-TDI, 0.23g (1mmol) of tetraethyltin, and 0.08g of hydroquinone into a 250mL four-necked flask as a polymerization inhibitor 1.53 g (15 mmol) of ethylene glycol monoallyl ether was added dropwise. After the addition was completed, TLC was used to detect the reaction progress, and the temperature was kept and stirred for 4 hours. 9.17 g (15 mmol) of N-methyl-N-hydroxyethyl-4-perfluorononenyloxybenzylamine was added dropwise. After the dropwise addition was completed, TLC was detected to track the reaction progress, and the temperature was kept stirring for 5 hours. Reaction is completed, and solvent extraction is separated by silica gel column chromatography (V 环己烷 :V 乙酸乙酯 =7:3), after removing the solvent, a light yellow paste I was obtained 1 (n=9), yield 68.9%, purity 99.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com