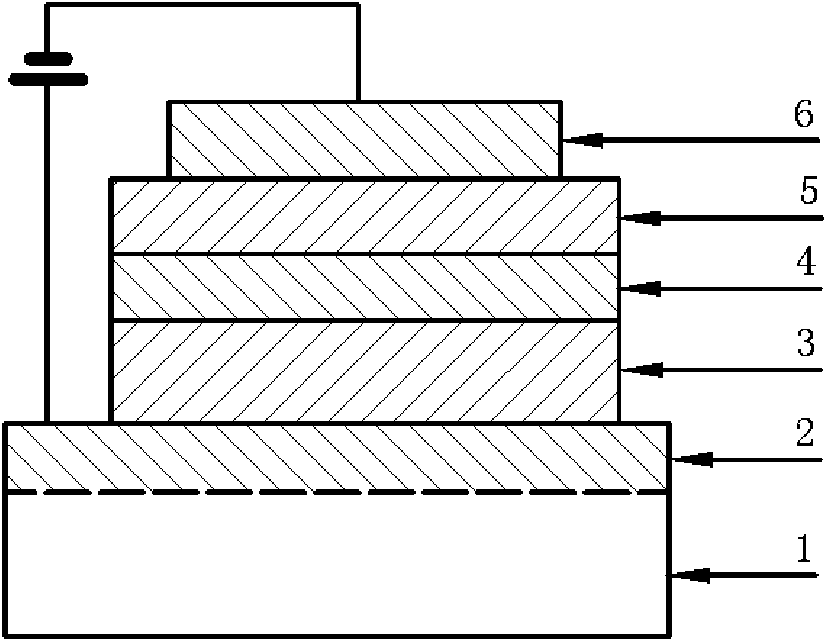
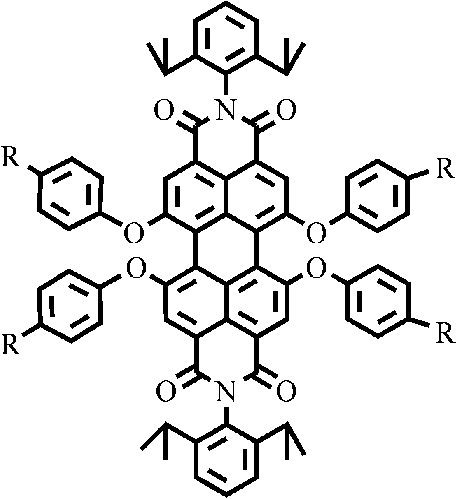
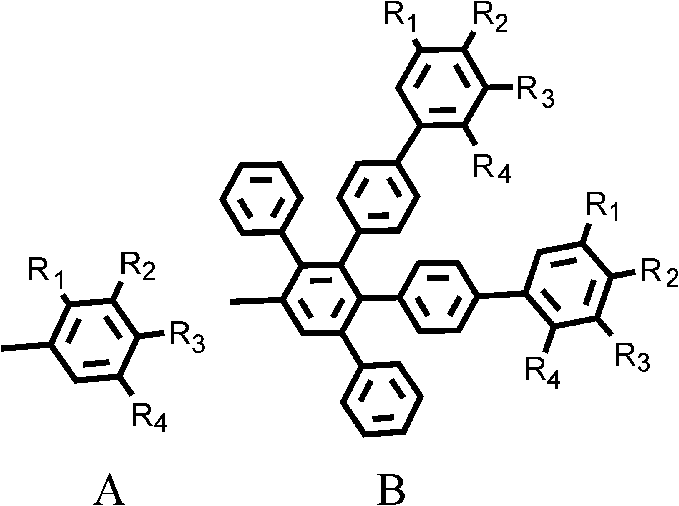
Perylene double imide derivatives and preparation method and applications thereof
A technology of perylene bisimide and derivatives, which is applied in the field of organic electroluminescent materials, can solve problems such as device instability, complicated device preparation process, and influence on the color purity of red light, and achieve photothermal stability and morphological stability High, high fluorescence quantum yield, good solubility effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] The preparation of embodiment 1 compound 4
[0021]
[0022] Add 1.2g (3.26mmol) 4,4'-dibromobenzophenone, 0.68g (3.26mmol) diphenylacetone, 200mg (3.59mmol) potassium hydroxide solid and 20ml absolute ethanol in a 50ml two-necked bottle, After reflux for 30 minutes, 1 (1.5 g, yield 80%) was obtained by suction filtration. 1 HNMR (400MHz, CDCl 3 )δ6.80(d, 4H, 3 J=8.4Hz), 7.1-7.3(m, 10H), 7.35(d, 4H, 3 J=8.4Hz). MS: m / z: 542 ([M+2] + ).
[0023]
[0024] Add 200mg (0.369mmol) 1, 178mg (0.811mmol) N-phenyl-1-naphthylamine, 118mg (1.84mmol) copper powder, 24.4mg (0.09mmol) 18-crown-6, 357mg ( 2.58 mmol) of potassium carbonate and 5 ml of nitrobenzene were reacted for 72 h under nitrogen protection under reflux, the solvent was removed under reduced pressure, and 2 (257 mg, yield 85%) was obtained by column chromatography. MS: m / z:: 819 ([M+H] + ).
[0025]
[0026] 135 mg (0.115 mmol) of intermediate 3 and 565 mg (0.69 mol) of 2 were refluxed in o-xylene; ...
Embodiment 2
[0027] The preparation of embodiment 2 compound 6
[0028]
[0029] 108 mg (0.09 mmol) of 3 and 548 mg (0.73 mmol) of 5 were refluxed in o-xylene for 5 days under nitrogen protection. Then it was cooled to room temperature, the solvent was removed under reduced pressure, and the target compound 6 (300 mg, 81%) was separated by column chromatography. Mp.413°C. 1 HNMR (CD 2 Cl 2 ): 8.17(s, 4H), 7.78(s, 4H), 7.44-7.38(t, 2H), 7.26-7.23(d, 4H), 7.09-7.05(d, 8H), 6.77-6.73(d, 8H ), 2.66-2.55(m, 4H), 1.03-1.00(s, 24H). 19 FNMR (CD 2 Cl 2 ): δ=-136.932~-138.826(m), -148.818~-149.007(d), -149.628(s), -150.527(s), -158.745~-159.332(m).MS(MALDI-TOF): m / z: 4039 ([M+H] + ).Elemental analysis calcd.(%)for C 192 h 58 f 80 N 2 o 8 : C, 57.07, H, 1.45, N, 0.69. Found: C, 57.01, H, 1.38, N, 0.73.
Embodiment 3
[0030] The preparation of embodiment 3 compound 8
[0031]
[0032] Add 45mg (0.038mmol) intermediate 3 and 215mg (0.228mmol) 7 in the 50ml two-necked flask, reflux in o-xylene under nitrogen protection for four days, then cool to room temperature, remove the solvent under reduced pressure, and separate by column chromatography to obtain 8 (120 mg, 65% yield). MS (MALDI-TOF): m / z: 4817 ([M+H] + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com