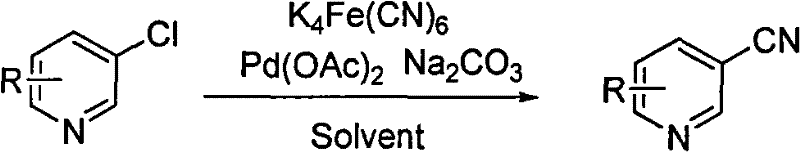Method for preparing cyanopyridine by using chloropyridine under the catalysis of ligand-free palladium
A technology of chloropyridine and cyanopyridine, which is applied in the field of preparing cyanopyridine from chloropyridine under the catalysis of ligand-free palladium, can solve the problems of high temperature and high pressure, limited sources of picoline, etc., and achieve the reduction of post-treatment process, The effect of reducing the harm of waste acid and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Potassium ferrocyanide trihydrate (557mg, 1.32mmol), 3-chloropyridine (678mg, 6mmol), potassium carbonate (828mg, 6mmol), palladium acetate (20mg, 0.03mmol) were dissolved in 100mL N, N-dimethylethyl Amide, nitrogen protection. Heat to 120°C for 3 hours. After the reaction, the temperature returned to room temperature, and the reaction solution was diluted with 100 mL of ethyl acetate. Filter, and wash the filtrate twice with 80 mL of water and 5% ammonia water respectively. The organic phase was dried over anhydrous magnesium sulfate. The volatile substances were removed by rotary evaporation to obtain 524 mg of the product 3-cyanopyridine with a yield of 84%. The conversion of 3-chloropyridine was 90%.
Embodiment 2
[0025] Potassium ferrocyanide (736mg, 2mmol), 2-chloro-3-picoline (1276mg, 10mmol), potassium carbonate (276mg, 2mmol), palladium acetate (33.5mg, 0.05mmol) were dissolved in 200mL 1,4- Dioxane, argon protection. Heat to 100°C for 1 hour. After the reaction, the temperature returned to room temperature, and the reaction solution was diluted with 200 mL of chloroform. Filter, and wash the filtrate twice with 150 mL of water and 5% ammonia water respectively. The organic phase was dried over sodium sulfate. The volatile substances were removed by rotary evaporation to obtain 932 mg of the product 2-cyano-3-methylpyridine with a yield of 79%. The conversion of 2-chloro-3-picoline was 95%.
Embodiment 3
[0027] Potassium ferrocyanide (3680mg, 10mmol), 2-chloro-3,5-lutidine (1416mg, 10mmol), potassium carbonate (1380mg, 10mmol), palladium acetate (67.4mg, 0.1mmol) were dissolved in 300mL di Methyl sulfoxide, nitrogen protection. Heat to 135°C for 4 hours. After the reaction, the temperature returned to room temperature, and the reaction solution was diluted with 300 mL of ethyl acetate. Filter, and wash the filtrate twice with 200 mL of water and 5% ammonia water respectively. The organic phase was dried over anhydrous magnesium sulfate. The volatile substances were removed by rotary evaporation to obtain 990 mg of the product 2-cyano-3,5-lutidine with a yield of 75%, and the conversion rate of 2-chloro-3,5-lutidine was 82%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com