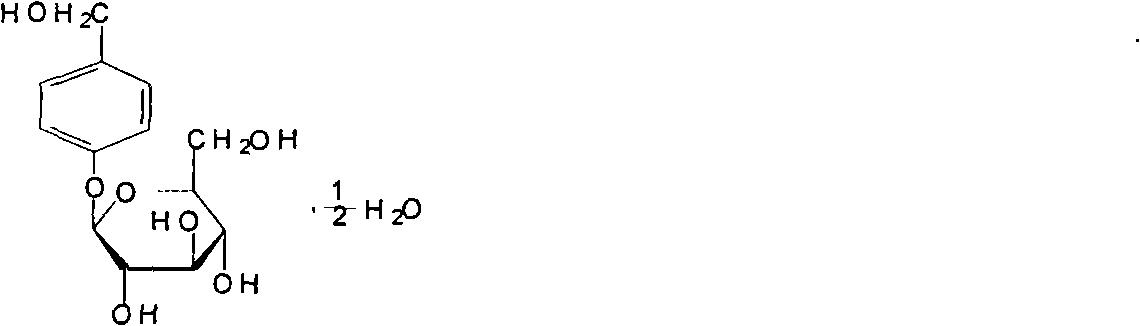Gastrodin injection and preparation method thereof
A technology for gastrodin injection and injection, which can be applied in pharmaceutical formulations, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc., which can solve injection pain or other adverse reactions and increase the cost of gastrodin injection. , the instability of gastrodin aqueous solution, etc., to achieve the effect of reducing unpredictable risks, good product stability, and reducing injection pain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1 1000
[0024] Prescription: Gastrodin 100g
[0025] Acetic acid-sodium acetate buffer 15ml
[0026] Add water for injection to 1000ml
[0027]
[0028] Made 1000 pieces
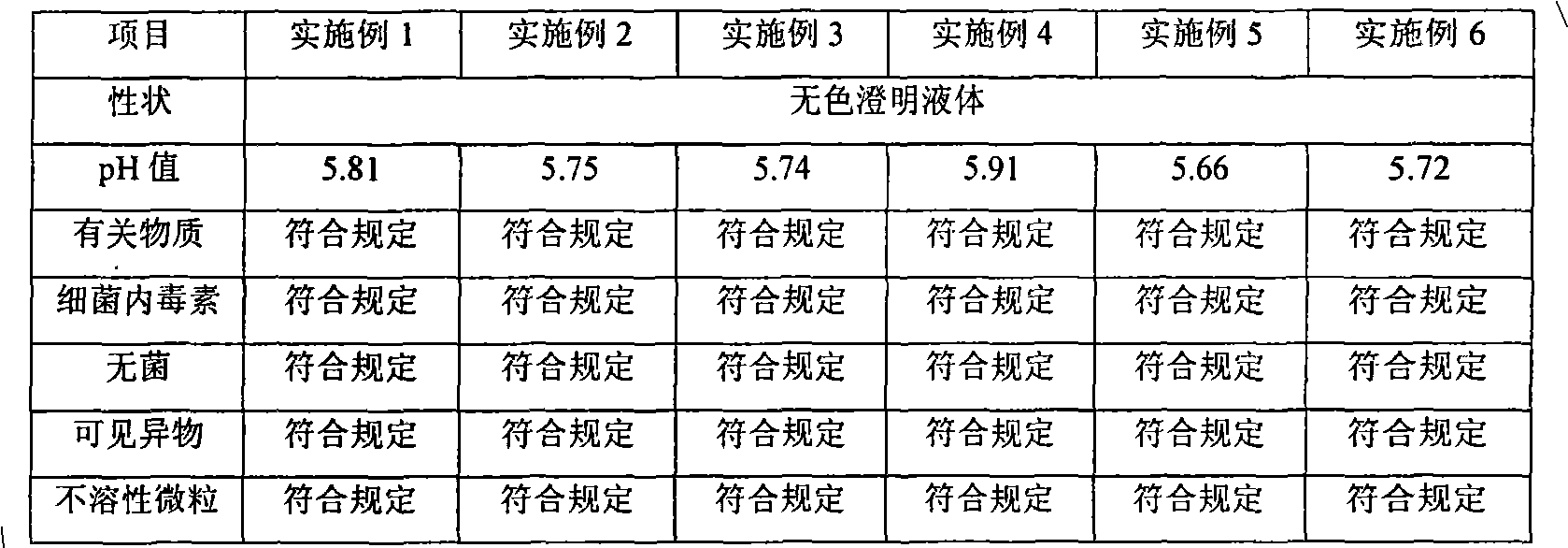
[0029] Prepare an appropriate amount of acetic acid-sodium acetate buffer solution for later use. Take an appropriate amount of water for injection and put it into the concentrated preparation tank, add 100g of gastrodin, and stir until completely dissolved. Add the prepared acetic acid-sodium acetate buffer solution, stir and mix evenly. Weigh 0.2% of the liquid amount of activated carbon into the concentrated preparation tank, add water for injection to the full amount, stir for 20 minutes, then circulate and stir for 10 minutes, filter for decarbonization, and then filter and sterilize through a 0.22 μm microporous filter element. The fine filtrate was taken for intermediate detection. After qualified, potting, autoclaving at 121°C for 15 min...
Embodiment 2
[0030] Embodiment 2 1000
[0031] Prescription: Gastrodin 100g
[0032] Citric acid 0.15g
[0033] Disodium hydrogen phosphate 0.36g
[0034] Add water for injection to 1000ml
[0035]
[0036] Made 1000 pieces
[0037] First take an appropriate amount of water for injection and put it into the concentrated preparation tank, add 100g of gastrodin, and stir until it is completely dissolved. Weigh the prescribed amount of citric acid and disodium hydrogen phosphate and dissolve it in an appropriate amount of water for injection. After stirring to dissolve, add it to the concentrated preparation tank and stir evenly. Weigh 0.2% of the liquid amount of activated carbon and add it to the concentrated preparation tank, add water for injection to the full amount, stir for 40 minutes, then circulate and stir for 10 minutes, filter for decarbonization, and then filter and sterilize through a 0.22 μm microporous filter element. The fine filtrate was ta...
Embodiment 3
[0038] Embodiment 3 1000
[0039] Prescription: Gastrodin 100g
[0040] Disodium hydrogen phosphate-sodium dihydrogen phosphate buffer 10ml
[0041] Acetic acid-sodium acetate buffer 10ml
[0042] Add water for injection to 1000ml
[0043]
[0044] Made 1000 pieces
[0045] Prepare appropriate amount of disodium hydrogen phosphate-sodium dihydrogen phosphate buffer and acetic acid-sodium acetate buffer for later use. Take 100g of water for injection and put it into the concentrated preparation tank, add 100g of gastrodin, and stir until completely dissolved. Add the prepared disodium hydrogen phosphate-sodium dihydrogen phosphate buffer and acetic acid-sodium acetate buffer, stir and mix evenly. Weigh 0.2% of the liquid amount of activated carbon and add it to the concentrated preparation tank, add water for injection to the full amount, stir for 30 minutes, then circulate and stir for 15 minutes, filter for decarbonization,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com