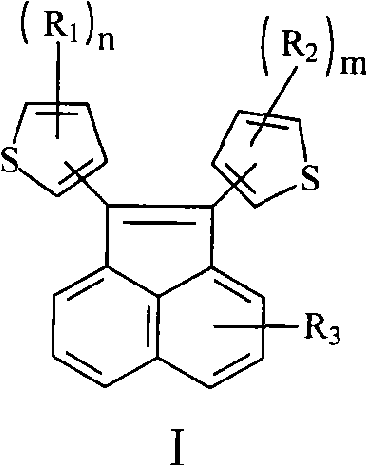
Acenaphthylene derivative
A technology of derivatives, acenaphthylene, applied in the field of acenaphthylene derivatives, can solve the problems of increasing costs, various parameters, harsh reaction conditions, etc., and achieve the effect of simple synthesis route and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
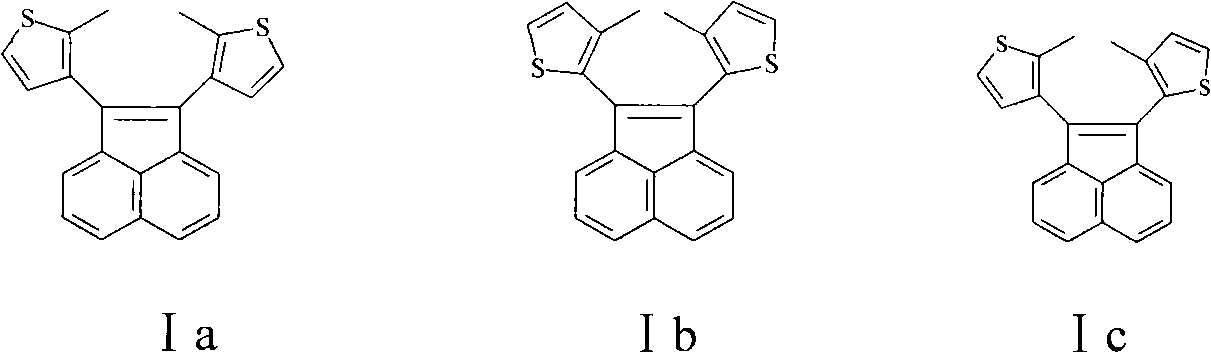
[0027] Preparation of 1,2-bis(3-methyl-2-thienyl)acenaphthylenene.
[0028] 6.2 g of the haloacenaphthylene compound and the catalyst were added to 50 ml of ether, and a reagent made of 2-halo-3-methylthiophene and magnesium powder was added dropwise at room temperature. After the dropwise addition was completed, the reaction was carried out for 12 hours. Dilute hydrochloric acid was poured into the reaction mixture to terminate the reaction. The organic phase was separated and the aqueous phase was extracted with ether (3 x 25ml). The organic phases were combined and washed with water. After drying over anhydrous magnesium sulfate, diethyl ether was removed by rotary evaporation. The residue was separated through a short silica gel column with petroleum ether as the eluent to obtain 5.1 g of solid with a yield of about 74%.
[0029] H NMR spectrum of the product: 1 H-NMR (500MZ, CDCl 3 , ppm) δ H 7.90 (m, 4H), 7.65 (m, 2H), 7.37 (d, 2H), 6.98 (d, 2H), 1.99 (s, 6H). Ma...
Embodiment 2
[0031] Synthesis of 1,2-bis(3,5-dimethyl-2-thienyl)acenaphthylenene.
[0032] 3,5-Dimethyl-2-thiopheneboronic acid (20mmol) and 1,2-dihaloacenaphthylenene (10mmol) were respectively added into a 100mL one-necked flask. Use tetrahydrofuran as a solvent for the reaction; and add a catalyst. Argon vacuum replaced the system three times, and refluxed overnight in an argon atmosphere in the dark. Pour into water, separate the layers, extract the inorganic phase with ether, combine the organic layers, and concentrate. Using petroleum ether and dichloromethane as the eluent, pass through a silica gel column. An orange-red solid was obtained in 81% yield.
[0033] H NMR spectrum of the product: 1 H-NMR (500MZ, CDCl 3 , ppm) δ H 7.81 (d, 2H), 7.64 (d, 2H), 7.55 (m, 2H), 6.60 (s, 2H), 2.41 (s, 6H), 2.22 (s, 6H). Mass spectrometry: EI-MS, M + / e:372.4
Embodiment 3
[0035] Preparation of 1,2-bis(3,4,5-trimethyl-2-thienyl)acenaphthylenene.
[0036] Add 20 mmol of dihaloacenaphthylene compound and catalyst together into 50 mL of ether, and dropwise add an organic reagent made of 2-halogenated 3,4,5-trimethylthiophene and magnesium powder. After the dropwise addition was completed, the reaction was carried out overnight. Dilute hydrochloric acid was poured into the reaction mixture to terminate the reaction. The organic phase was separated and the aqueous phase was extracted three times with ether. The organic phases were combined and washed with water. After drying over anhydrous magnesium sulfate, diethyl ether was removed by rotary evaporation. The residue was separated through a short silica gel column with petroleum ether as the eluent to obtain a pure product with a yield of about 69%.
[0037] Proton NMR spectrum: 1 H-NMR (500MZ, CDCl 3 , ppm) δ H 7.91 (d, 2H), 7.74 (d, 2H), 7.59 (m, 2H), 2.25 (s, 6H), 2.41 (s, 6H), 2.12 (s, 6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com