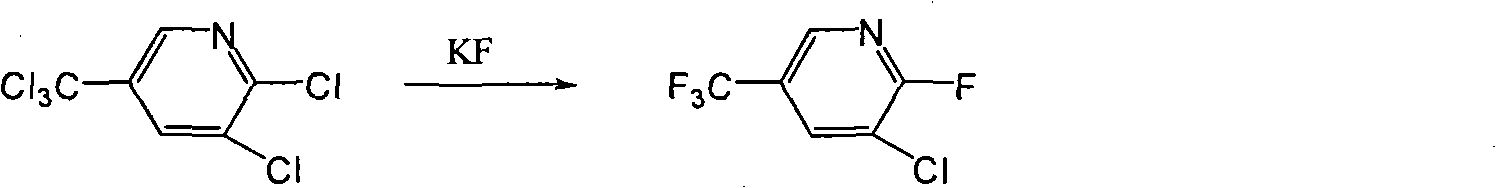
Novel method for synthesizing 2-fluoro-3-chloro-5-trifluoromethylpyridine
A technology for trifluoromethylpyridine and trichloromethylpyridine, which is applied in the field of synthesizing 2-fluoro-3-chloro-5-trifluoromethylpyridine, can solve the problems of unsatisfactory yield and general effect, and achieves Environmentally friendly, enhanced molecular activity, mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] 25g (0.43mol) of potassium fluoride, 50ml of toluene and 150ml of N, N-dimethylformamide were added to a 250ml four-necked reaction flask, and 0.66g (0.0029mol) of benzyltriethylammonium chloride was added as a phase transfer catalyst. Install the steam-water separator, heat to reflux at 115°C for reflux and dehydration for 3 hours, after the dehydration is completed, add 19.1g (0.072mol) of 2,3-dichloro-5-trichloromethylpyridine, and keep it warm at 120°C for 5 hours. After the end, filter with suction and discard the solid phase. The organic phase was decompressed and rectified to collect the effluent at 50-55°C / 11mmHg to obtain 2-fluoro-3-chloro-5-trifluoromethylpyridine with a purity of 98.5% and a yield of 94.7%.
Embodiment 2
[0024] Except that the reaction time was 6 hours, the ratio of potassium fluoride to 2,3-dichloro-5-trichloromethylpyridine added was 8:1, other reaction conditions and post-treatment methods were the same as in Example 1, and finally 2 -Fluoro-3-chloro-5-trifluoromethylpyridine, the purity reached 95.2%, and the yield was 92.1%.
Embodiment 3
[0026] Except that the reflux dehydration temperature was 90°C, other reaction conditions and post-treatment methods were the same as in Example 1, and finally 2-fluoro-3-chloro-5-trifluoromethylpyridine was obtained with a purity of 94.2% and a yield of 89% %.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com