Sodium salt compound of Iguratimod, preparation method thereof and pharmaceutical use thereof
A potassium compound and reaction technology, applied in the field of Iilamod's derivative compounds, can solve the problems of degradation, poor fluidity, and poor stability of Iilamod.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
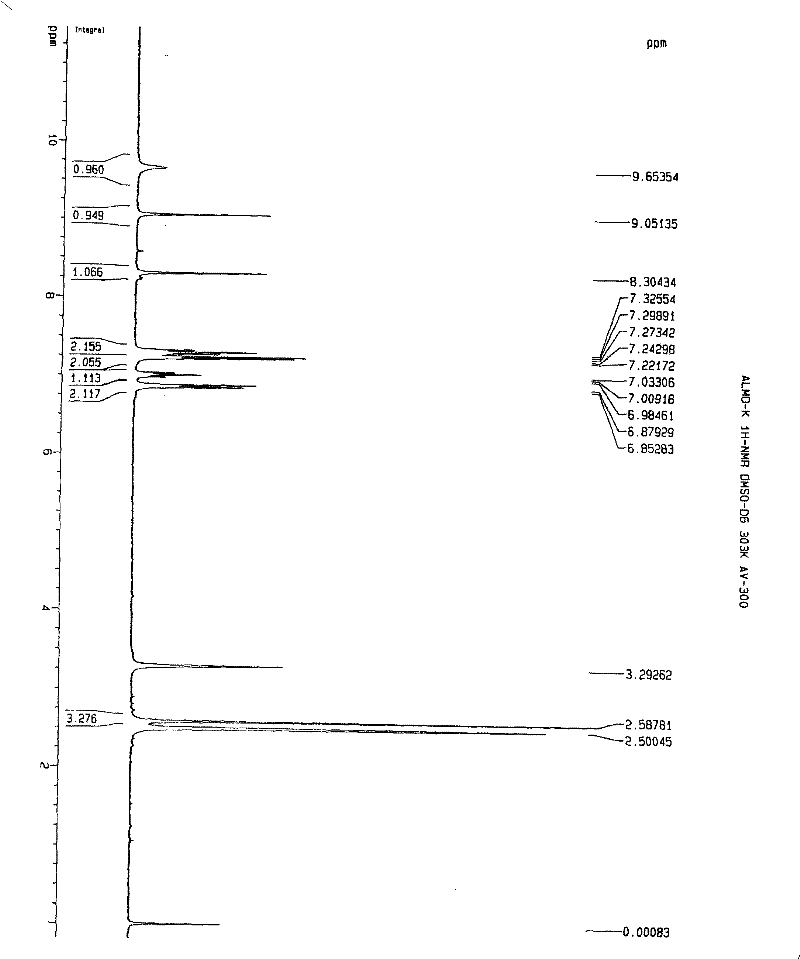
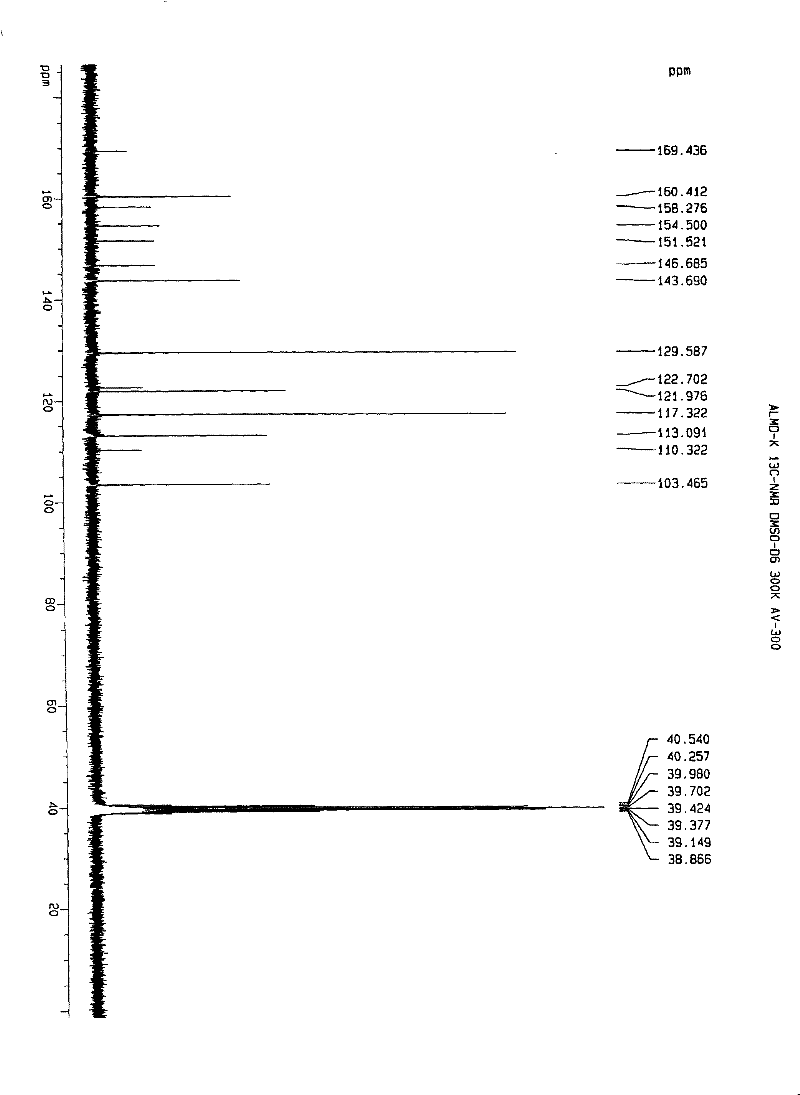
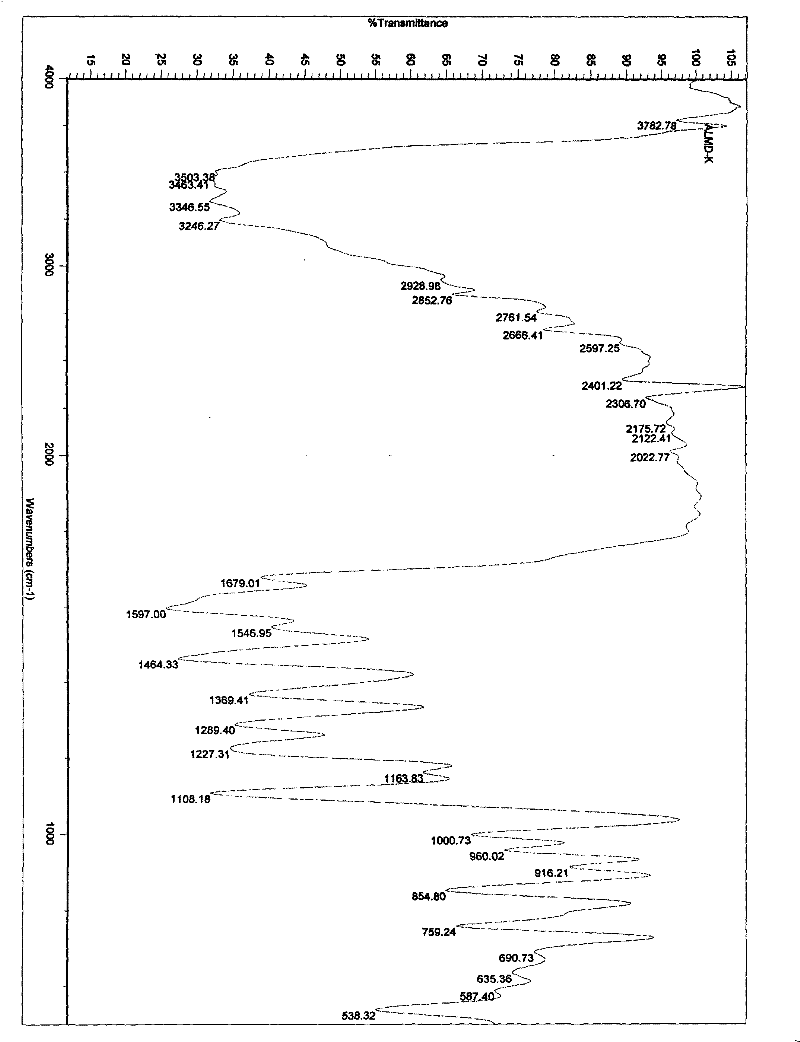
[0112] Embodiment 1. iguratimod potassium and its preparation
[0113] Method 1. Add 1 g (about 2.67 mmol) of iguratimod to 400 ml of absolute ethanol, heat to reflux to dissolve, then add 150 mg of potassium hydroxide (about 2.67 mmol) in 10 ml of absolute ethanol, stir and mix at 80°C , reaction, if necessary, 280mg of activated carbon can be added, stirred and kept for decolorization and filtered out activated carbon, the filtrate is spin-dried with a rotary evaporator to remove the solvent, and the solid obtained is washed or rinsed repeatedly with cold ethanol or ether, the washing solvent is removed, and dried, to obtain Airamod Potassium. Molecular formula C 17 h 13 N 2 o 6 SK, elemental analysis results:
[0114] % Theoretical C: 49.50H: 3.18N: 6.79
[0115] % Found C: 49.56H: 3.22N: 6.81.
[0116] Method 2. Take 1g (about 2.67mmol) of iguratimod and add 80ml of distilled water to stir to make a suspension, and add 151mg of potassium hydroxide (about 2.7mmol) in...
Embodiment 2
[0126] Embodiment 2. iguratimod potassium and its preparation
[0127] Method 1. Take 1 g (about 2.67 mmol) of iguratimod and add 200 ml of acetonitrile, heat to reflux to dissolve, then add 151 mg of potassium hydroxide (about 2.7 mmol) in 10 ml of absolute ethanol, stir and mix at 70 ° C, Reaction, if necessary, add an appropriate amount of activated carbon, stir and keep warm for decolorization and filter out the activated carbon, spin the filtrate to remove the solvent with a rotary evaporator, and wash or rinse the solid matter repeatedly with cold ethanol or ether, remove the washing solvent, and dry to obtain Ai. Lamod Potassium.
[0128] Method 2. Take 1 g (about 2.67 mmol) of iguratimod and add 300 ml of absolute ethanol, heat to reflux to dissolve, then add a solution formed by 227 mg of potassium ethylate (about 2.7 mmol) in 17 ml of ethanol, mix and react at room temperature, add 0.15% (W / V) amount of activated carbon, heated and stirred at 55-90°C for 20 minutes,...
Embodiment 3
[0129] Embodiment 3. iguratimod potassium and its preparation
[0130] Take 1g of iguratimod (about 2.67mmol) and add 160ml of acetone to heat and reflux to dissolve, then add dropwise the solution formed by 150mg of potassium hydroxide (about 2.67mmol) in 2ml of distilled water, after adding it completely, keep warm and stir to mix and react until it drops to Suction filter at room temperature, wash the filter cake with acetone 2 to 4 times, and dry to obtain iguratimod potassium and its hydrate.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com