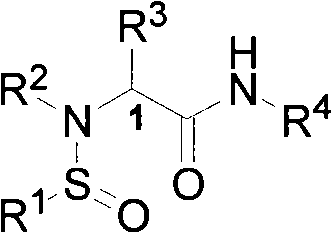
N-sulfinyl amino acid amide compound and application thereof
A sulfinyl amino acid amide and compound technology, which is applied in the field of optically pure N-sulfinyl amino acid amide compounds to achieve good stereoselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: the preparation of compound 1, structural formula is as follows:
[0027]
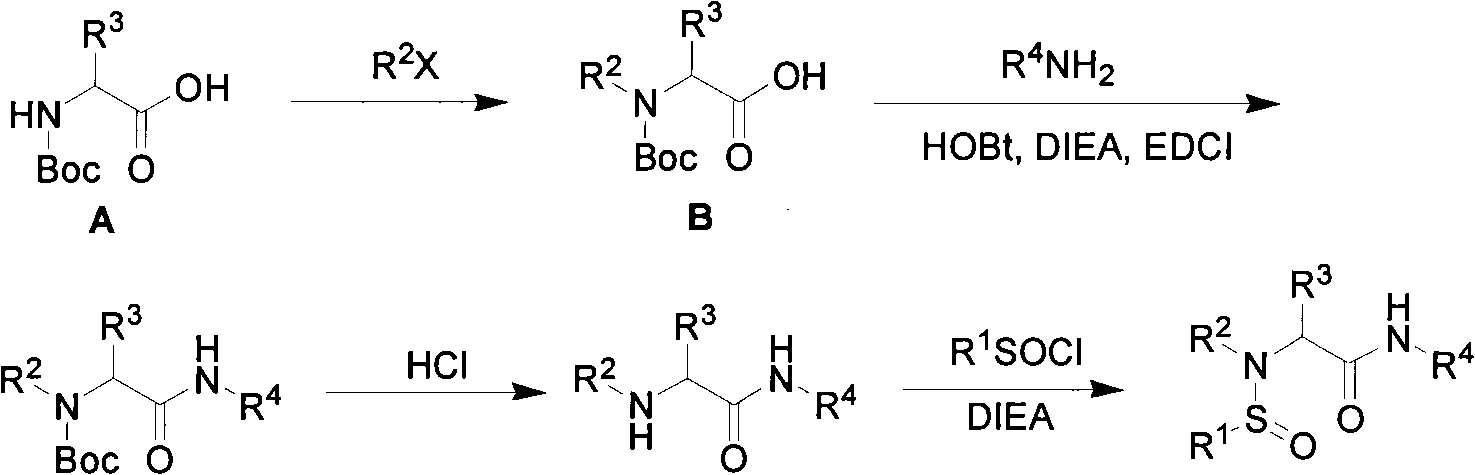
[0028] In a 100mL round bottom flask, add tetrahydrofuran (30mL), under argon protection, add N-Boc valine (1.3g, 6mmol), methyl iodide (9.1mL, 60mmol) and sodium hydride (2.53g, 60mmol) at zero . After the reaction was completed, add water to quench, evaporate the solvent under reduced pressure, add 50 mL of water to dissolve the residue, extract with ether, discard the organic phase, adjust the pH value of the aqueous phase to 3 with 1N hydrochloric acid, extract with ethyl acetate, combine the organic phase, After drying over magnesium sulfate, the solvent was evaporated under reduced pressure to obtain N-Boc-N-methylvaline as a colorless oil with a yield of 97%.
[0029] In a 50 mL round bottom flask, add the above colorless oil (200 mg, 0.86 mmol) and dichloromethane (5 mL), then add aniline (0.94 mL, 1.04 mmol), HOBt (153 mg, 1.04 mmol), DIEA (0.36 mL, 2.08mmol), EDCI (2...
Embodiment 2
[0032] Embodiment 2: the preparation of compound 2, structural formula is as follows:
[0033]
[0034] In a 50mL round bottom flask, add N-Boc-N-methylvaline (947mg, 4.1mmol) and dichloromethane (20mL), then add amantadine (500mg, 2.7mmol), HOBt (657.9mg , 4.05mmol), DIEA (1.12mL, 6.5mmol), EDCI (614mg, 3.2mmol). The reaction conditions, post-treatment method and subsequent removal of N-Boc protecting group and N-tert-butyl sulfonylation were the same as in Example 1 to obtain compound 2 as a white solid with a yield of 75%. 1 H NMR (300MHz, CDCl 3 ): δ (ppm) 6.07 (s, 1H), 3.26 (d, J = 8.76, 1H), 2.63 (s, 3H), 2.36-2.29 (m, 1H), 2.03 (s, 9H), 1.66 (s , 8H), 1.24(s, 9H), 0.90(d, J=6.66, 6H); 13 C NMR (75MHz, CDCl 3 ): δ (ppm) 168.8, 74.1, 59.5, 52.2, 41.2, 36.3, 29.4, 27.9, 27.8, 24.1, 20.5, 19.8; ESI HRMS (C 20 h 36 N 2 o 2 S 1 +Na) + The theoretical value is m / z 391.2390, and the measured value is m / z 391.2386.
Embodiment 3
[0035] Embodiment 3: the preparation of compound 3, structural formula is as follows:
[0036]
[0037] In a 50mL round bottom flask, add N-Boc-N-methylvaline (1.0g, 4.3mmol) and dichloromethane (30mL), then add p-fluoroaniline (0.35mL, 3.61mmol), HOBt ( 690mg, 4.69mmol), DIEA (1.5mL, 8.66mmol), EDCI (920mg, 4.69mmol), the reaction conditions and post-treatment methods and the subsequent removal of N-Boc protecting group and N-tert-butyl sulfonylation are the same as in the examples 1. Compound 3 was obtained as a white solid with a yield of 70%. 1 H NMR (300MHz, CDCl 3 ): δ (ppm) 9.06 (bs, 1H), 7.57-7.51 (m, 2H), 6.99-6.93 (m, 2H), 4.36 (d, J=7.05, 1H), 2.67 (s, 3H), 2.54 -2.44 (m, 1H), 1.33 (s, 9H), 1.09 (d, J=6.57, 3H), 1.03 (d, J=5.88, 3H); ESI HRMS (C 16 h 25 f 1 N 2 o 2 S 1 +Na) + The theoretical value m / z 351.1513, the measured value m / z 351.1517.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com