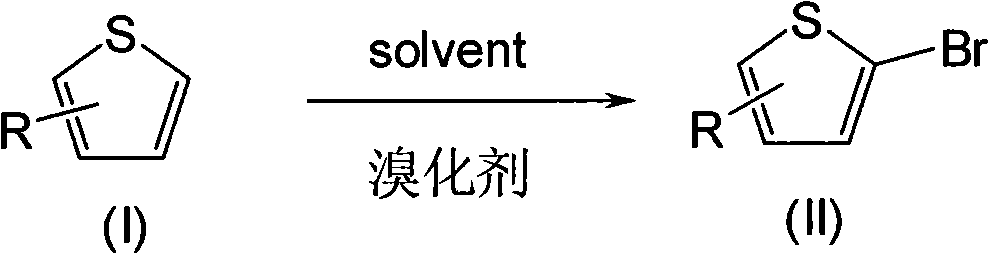
Chemical synthesis method for 2-bromothiophene and derivative thereof
A technology of chemical synthesis and synthesis method, which is applied in the field of chemical synthesis of 2-bromothiophene and its derivatives, can solve the problems of low product purity, low yield, poor reaction selectivity, etc., and achieve the improvement of reaction selectivity and reaction yield. The effect of increasing the efficiency and improving the purity of the product
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Add 95g (1.131mol) of thiophene and 300ml of chloroform into a 1000ml three-necked reaction flask, start stirring, cool to -5°C, slowly add about 320g (1mol) of pyridinium tribromide in batches under stirring, after adding, continue to keep warm React for 0.5 hours, then add about 100ml of water, stir for 0.5 hours, let stand to separate the water layer, concentrate the organic layer to dryness, and rectify the concentrated solution under reduced pressure to obtain 148g (0.908mol) of 2-bromothiophene, the yield is 90.8%, the content 99.5%.
Embodiment 2
[0028] Add thiophene 95g (1.131mol), n-hexane 300ml, 2-picoline 101g (1.0mol) and 48% hydrobromic acid aqueous solution 337.5g (2mol) to a 1000ml three-necked reaction flask, start stirring, cool to -10°C, Slowly add 97.1 g (1.0 mol) of 35% hydrogen peroxide dropwise under stirring. After the addition is complete, continue the heat preservation reaction for 0.5 hours, let stand to separate the water layer, concentrate the organic layer to dryness, and rectify the concentrated solution under reduced pressure to obtain 2-bromothiophene 138.5g (0.85mol), the yield is 85%, and the content is 98.8%.
Embodiment 3
[0030] Add 100g (1.1905mol) of thiophene, 300ml of dichloromethane, 79g (1.0mol) of pyridine and 48.6g (0.5mol) of 35% hydrogen peroxide into a 1000ml three-necked reaction flask, start stirring, cool to -10°C, and slowly Add 80 g (0.5 mol) of bromine dropwise. After the addition is complete, continue the heat preservation reaction for 0.5 hours, let stand to separate the water layer, concentrate the organic layer to dryness, and rectify the concentrated solution under reduced pressure to obtain 145 g (0.8896 mol) of 2-bromothiophene. The rate is 88.96%, and the content is 99.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com