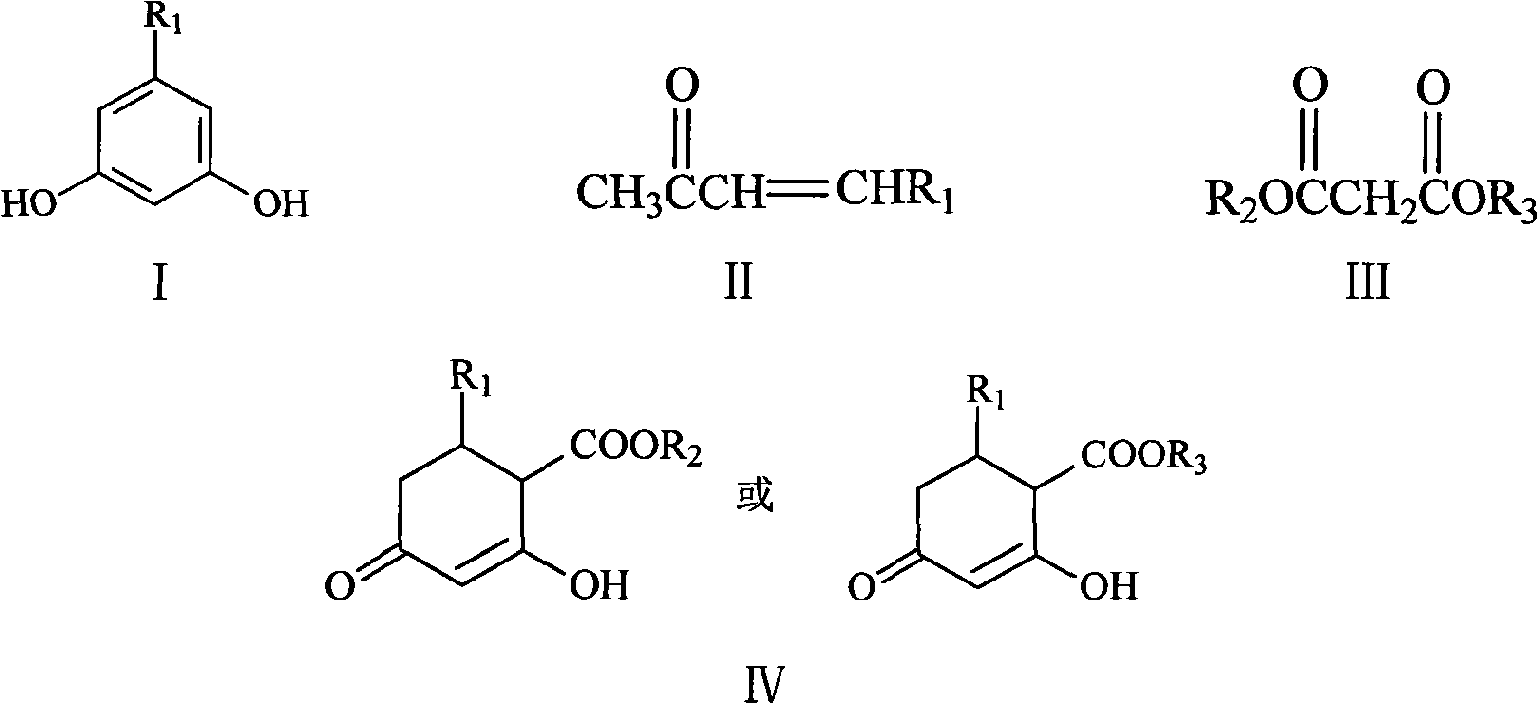
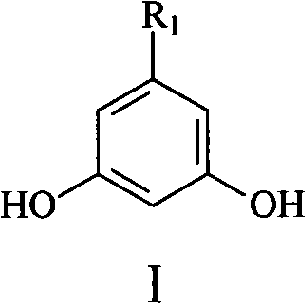
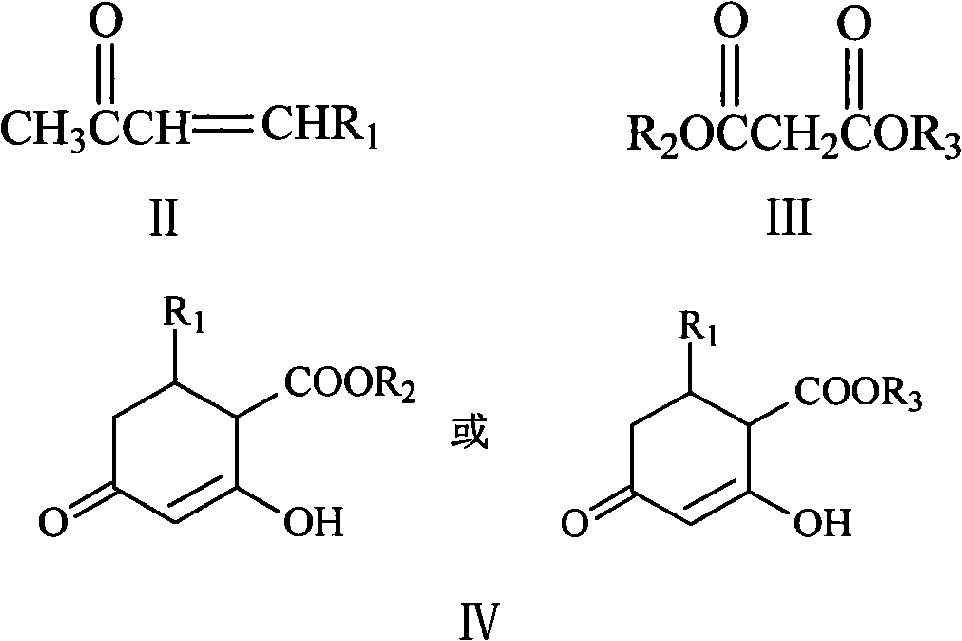
Method for synthesizing 5-alkyl-resorcin
A technology of resorcinol and alkyl, which is applied in the field of preparation of 5-alkylresorcinol, can solve the problems of high price, low yield, serious environmental pollution of reaction waste, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Mix 13ml of 2% NaOH aqueous solution with 40ml of acetone, stir at room temperature for 1 hour, then slowly add 20g of 40% acetaldehyde aqueous solution dropwise thereto, keep the temperature at 15-20°C, and react for 1.5 hours. Acetone was recovered by atmospheric distillation, and the residual liquid (20ml×3) was extracted with dichloromethane, dried over anhydrous sodium sulfate and concentrated to obtain orange-yellow liquid 4-hydroxy-2-pentanone. Add 20 g of 4-hydroxy-2-pentanone and 2 g of phosphoric acid into 18 ml of toluene, heat to reflux, react for 3 h, distill off the azeotrope of toluene and water under normal pressure, and then distill off the light yellow fraction under reduced pressure to obtain 3- Penten-2-one, dried over anhydrous sodium sulfate. The mixed solution of 3-penten-2-one 8.4g and dimethyl malonate 16.5g in N 2 Drop it into 65ml of 10% sodium methoxide / methanol solution under protection, raise the temperature to reflux, react for 3h, recove...
Embodiment 2
[0032] 5%K 2 CO 3 Mix 150ml of aqueous solution with 300ml of acetone, stir at room temperature for 1h, then slowly add 150g of 40% acetaldehyde aqueous solution to it dropwise, keep the temperature at 15-20°C, and react for 3h. Acetone was recovered by atmospheric distillation, and the residue (150ml×3) was extracted with dichloromethane, dried over anhydrous sodium sulfate and concentrated to obtain orange-yellow liquid 4-hydroxyl-2-pentanone, and 4-hydroxyl-2-pentanone Add 40g of sulfuric acid and 3g of toluene to 36ml of toluene, heat to reflux, react for 3h, distill the azeotrope of toluene and water under normal pressure, and then distill off the light yellow fraction under reduced pressure to obtain 3-penten-2-one, anhydrous Na2SO4 dried. The mixed solution of 3-penten-2-one 16g and dimethyl malonate 32g in N 2 Drop it into 130ml of 10% sodium methoxide / methanol solution under protection, heat up to reflux, react for 3h, remove methanol, dissolve the residue in 150ml...
Embodiment 3
[0035] 10% K 2 CO 3 Mix 50ml of aqueous solution with 200ml of acetone, stir at room temperature for 1 hour, then slowly add 80g of 40% acetaldehyde aqueous solution to it dropwise, keep the temperature at 15-20°C, and react for 2 hours. The acetone was recovered by atmospheric distillation, and the residue was extracted with dichloromethane (100ml×3), dried over anhydrous sodium sulfate and concentrated to obtain orange-yellow liquid 4-hydroxy-2-pentanone. Add 30g of 4-hydroxy-2-pentanone and 1g of DBU to 27ml of toluene, heat to reflux, react for 2h, distill the azeotrope of toluene and water under normal pressure, and then distill off the light yellow fraction under reduced pressure to obtain 3- Penten-2-one, dried over anhydrous sodium sulfate. The mixed solution of 24 g of 3-penten-2-one and 48 g of dimethyl malonate was placed in N 2 Drop it into 130ml of 10% sodium methoxide / methanol solution under protection, raise the temperature to reflux, react for 3h, recover me...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com