Method for preparing aromatic hydrocarbons through hydrogenation of carbon dioxide
A technology for carbon dioxide and aromatic hydrocarbons, which is applied in the production of hydrocarbons from carbon oxides, chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, etc. and high selectivity of methane, to achieve the effect of simple preparation method, good stability and simple reaction device
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] 15.81g FeCl 3 ·6H 2 O and 6.27 g FeCl 2 4H 2 O was mixed with 80 mL of water to form an iron salt solution, and 3.5 mL of 9.0 mol / L HCl solution was added. Add about 180 mL of 1.5 mol / L NaOH solution at a constant speed at 60°C under stirring conditions. Within about 1.5 h, the pH of the solution was adjusted from acidic to about 10. After the dropwise addition, keep the temperature and continue stirring for 1 h, and finally cool to room temperature. After the reaction, the deposited product was separated by magnetic field adsorption, washed once with 400mL deionized water, and then dried at 60°C to obtain Na-Fe 3 o 4 Catalyst samples, the samples are prepared after grinding, tableting and sieving.
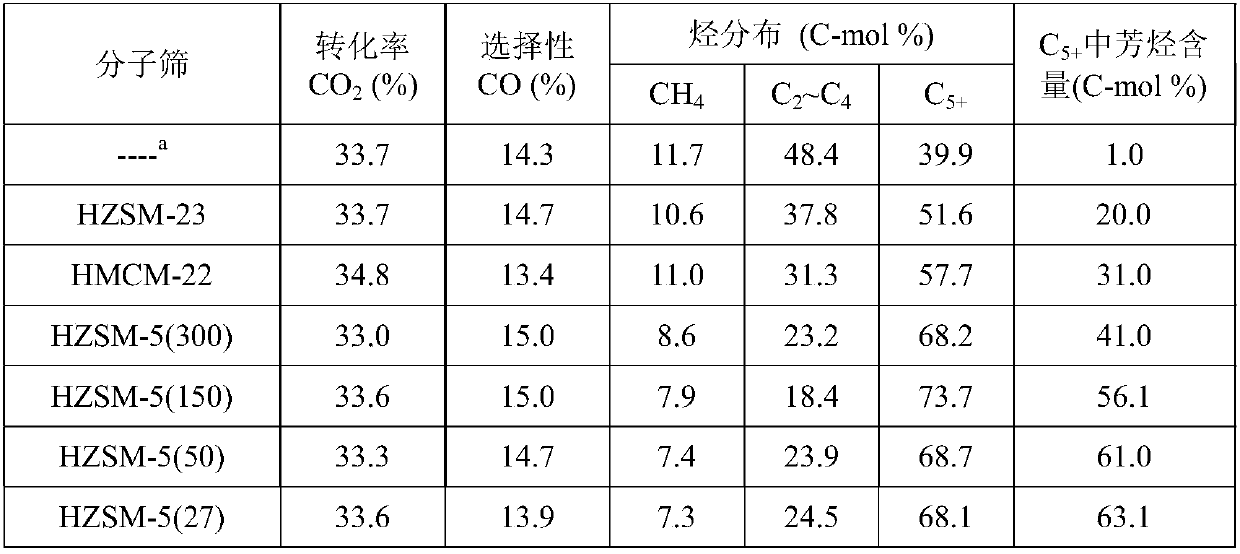
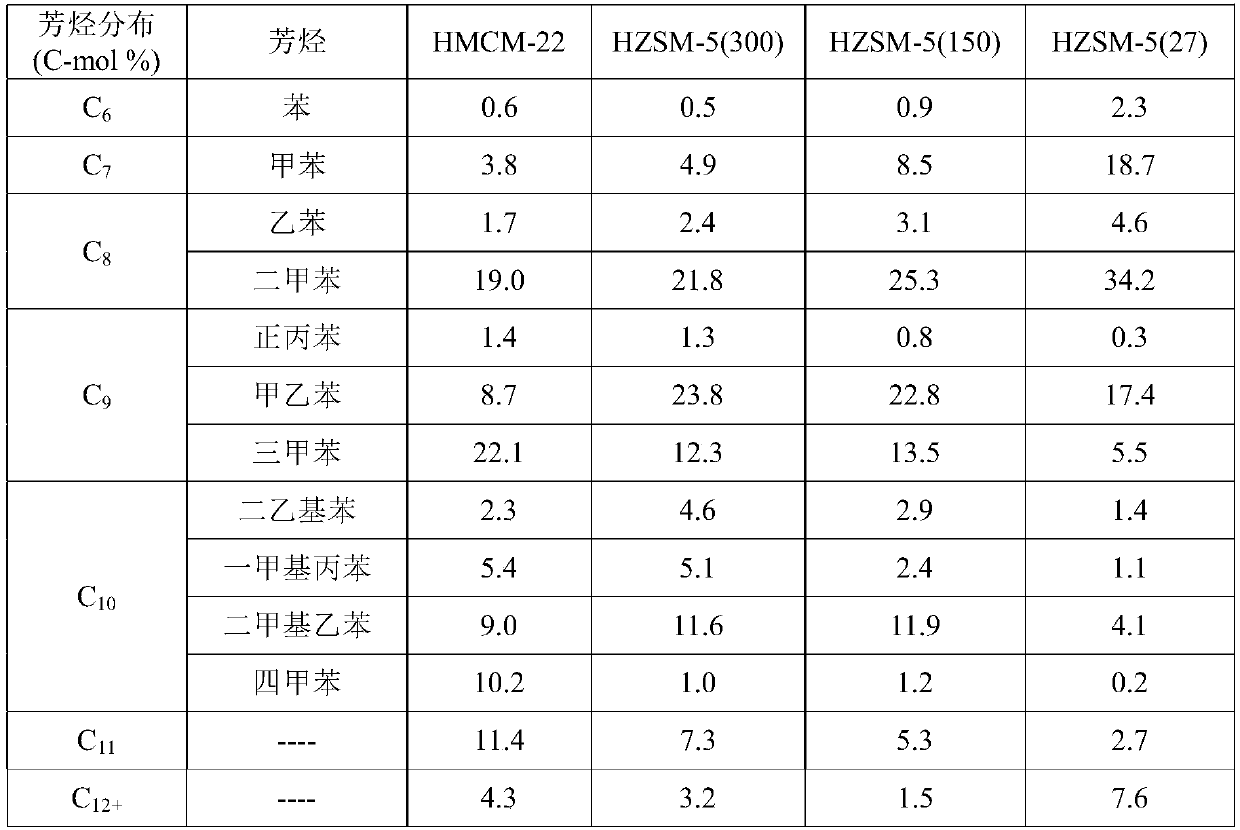
[0040] HMCM-22 (SiO 2 / Al 2 o 3 =30), silicon aluminum than SiO 2 / Al 2 o 3 HZSM-5 molecular sieves of 27, 50, 150, and 300, respectively, and HZSM-23 (SiO 2 / Al 2 o 3 =80) Molecular sieves were calcined at 500°C for 4 hours, and the samples were ground, pre...
Embodiment 2
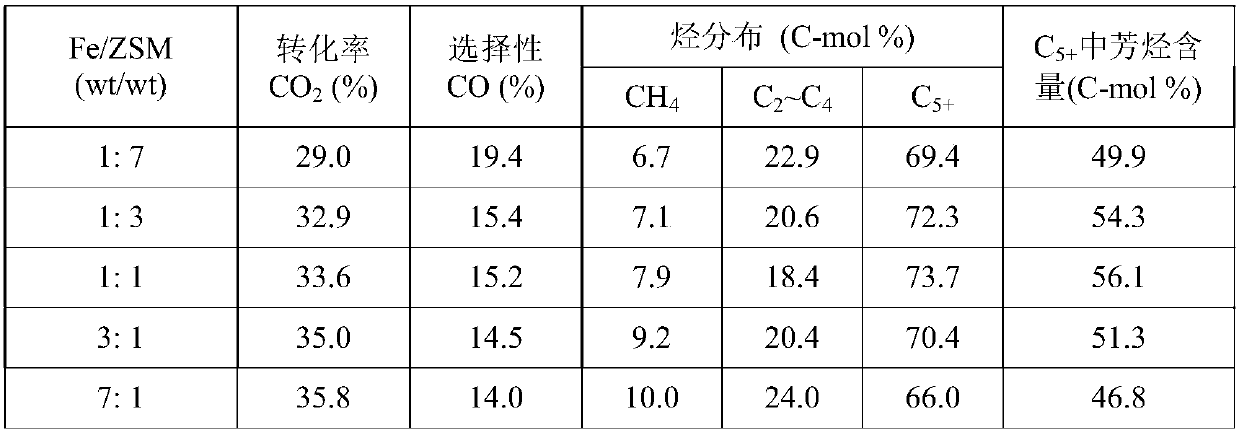
[0048] Take respectively the Na-Fe prepared by the method of Example 1 according to different mass ratios 3 o 4 Catalyst and HZSM-5 (SiO 2 / Al 2 o 3 =150) Molecular sieve, composed of a granular mixed catalyst with a total mass of 1.0g, mixed uniformly and used for CO 2 hydrogenation reaction. Reduction conditions: under normal pressure, pure H 2 (25mL / min), the reduction time is 8h at 350°C. Reaction condition: H 2 / CO 2 =3.0, the temperature is 320°C, the pressure is 3.0MPa, and the space velocity is 4000mL / (h·g cat ), investigated the mass ratio of two components to Na-Fe 3 o 4 / HZSM-5 Catalyst CO 2 The impact of hydrogenation performance, the results (see Table 3) show that the composite catalyst has dual functionality, and there is a synergistic effect between the two components. As the ratio of the two components changes, when the ratio of the two components Fe / ZSM is 1:1, the composite The catalyst has the best performance and the highest selectivity for aro...
Embodiment 3
[0052] Weigh respectively the Na-Fe prepared by 0.5g embodiment 1 method 3 o 4 Catalyst and 0.5g HZSM-5 (SiO 2 / Al 2 o 3 =150) Molecular sieve particles are mixed evenly and used for CO 2 hydrogenation reaction. Reduction conditions: under normal pressure, pure H 2 (25mL / min), the reduction time is 8h at 350°C. Reaction condition: H 2 / CO 2 =3.0, the temperature is 280~380°C, the pressure is 3.0MPa, the space velocity is 2000mL / (h·g cat ), investigated the effect of reaction temperature on Na-Fe 3 o 4 / HZSM-5 Catalyst CO 2 The impact of hydrogenation performance, the results (see Table 4) show that as the reaction temperature increases, CO 2 The conversion rate increases gradually, C 5+The selectivity increased first and then decreased, and the catalyst consistently exhibited excellent CO 2 Aromatization properties.
[0053] Table 4 reaction temperature to Na-Fe 3 o 4 / HZSM-5(150) composite catalyst on CO 2 Effect of hydrogenation performance
[0054]
PUM
| Property | Measurement | Unit |
|---|---|---|
| composition ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com