Stable chemical nickel-plating plating solution and preparation method thereof
A technology of electroless nickel plating and plating solution, which is applied in liquid chemical plating, metal material coating process, coating, etc. The effect of preventing the service life from being too low and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
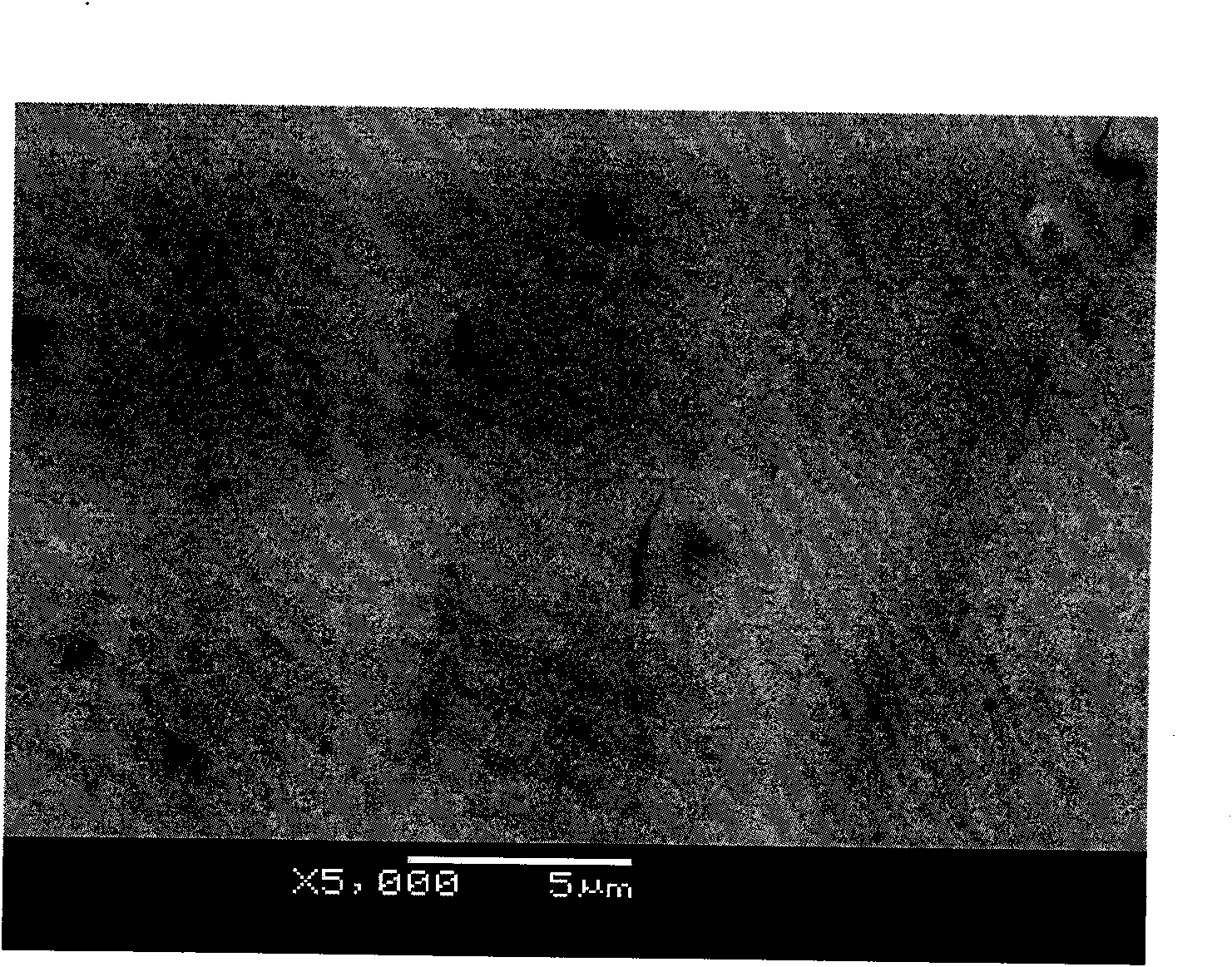
Image
Examples
Embodiment 1
[0025] 1. Take a 1L beaker and put in 500ml of water; weigh 6g of sodium carbonate into the beaker and stir to dissolve completely;
[0026] 2. Pour 5 grams of succinic acid dissolved in water into the beaker and stir evenly;
[0027] 3. Pour 16ml of lactic acid solution into a beaker and stir evenly, then use sodium carbonate to adjust the Ph=4 of the plating solution;
[0028] 4. Weigh 5 grams of citric acid to dissolve it, pour it into a beaker and stir evenly;
[0029] 5. Pour 2ml of propionic acid into a beaker and stir evenly;
[0030] 6. Pour 5g of malic acid dissolved in water into the beaker and stir evenly;
[0031] 7. Weigh 30g of sodium hypophosphite and pour it directly into a beaker to dissolve it completely and stir evenly.
[0032] 8. Weigh 30g of nickel sulfate and pour it directly into the beaker to make it completely dissolve and stir evenly;
[0033] 9. Weigh 4 mg of thiosemicarbazide that has been completely dissolved in water and pour it into a beaker...
Embodiment 2
[0038] 1. Take a 1L beaker and put in 500ml of water, weigh 5g of sodium carbonate into the beaker and stir to dissolve completely;
[0039] 2. Pour 3 grams of succinic acid dissolved in water into the beaker and stir evenly;
[0040] 3. Pour 10ml of lactic acid solution into a beaker and stir evenly, then use sodium carbonate to adjust the Ph=4 of the plating solution;
[0041] 4. Weigh 15 grams of citric acid to dissolve it, pour it into a beaker and stir evenly;
[0042] 5. Pour 4ml of propionic acid into a beaker and stir evenly;
[0043] 6. Pour 12g of malic acid dissolved in water into the beaker and stir evenly;
[0044] 7. Weigh 35g of sodium hypophosphite and pour it directly into a beaker to dissolve it completely and stir evenly.
[0045] 8. Weigh 32g of nickel sulfate and pour it directly into the beaker to make it completely dissolve and stir evenly;
[0046] 9. Weigh 4 mg of thiosemicarbazide that has been completely dissolved in water and pour it into a beak...
Embodiment 3
[0051] 1. Take a 1L beaker and put in 500ml of water, weigh 4g of sodium carbonate into the beaker and stir to dissolve completely;
[0052] 2. Pour 8 grams of succinic acid dissolved in water into the beaker and stir evenly;
[0053] 3. Pour 20ml of lactic acid solution into a beaker and stir evenly, then use sodium carbonate to adjust the Ph=4 of the plating solution;
[0054] 4. Weigh 15 grams of citric acid to dissolve it, pour it into a beaker and stir evenly;
[0055] 5. Pour 6ml of propionic acid into a beaker and stir evenly;
[0056] 6. Pour 19g of malic acid dissolved in water into the beaker and stir evenly;
[0057] 7. Weigh 35g of sodium hypophosphite and pour it directly into a beaker to dissolve it completely and stir evenly.
[0058] 8. Weigh 35g of nickel sulfate and pour it directly into the beaker to make it completely dissolve and stir evenly;
[0059] 9. Weigh 6 mg of thiosemicarbazide that has been completely dissolved in water, pour it into a beaker ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com