Matrine sustained release preparation and preparing method thereof
A technology of matrine and sustained-release tablets, which is applied in pill delivery, pharmaceutical formulations, antiviral agents, etc., can solve problems such as inconvenience, inconvenient medication, and obvious pain, and achieve high bioavailability, sufficient drug release, and blood drug Concentration stabilization effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
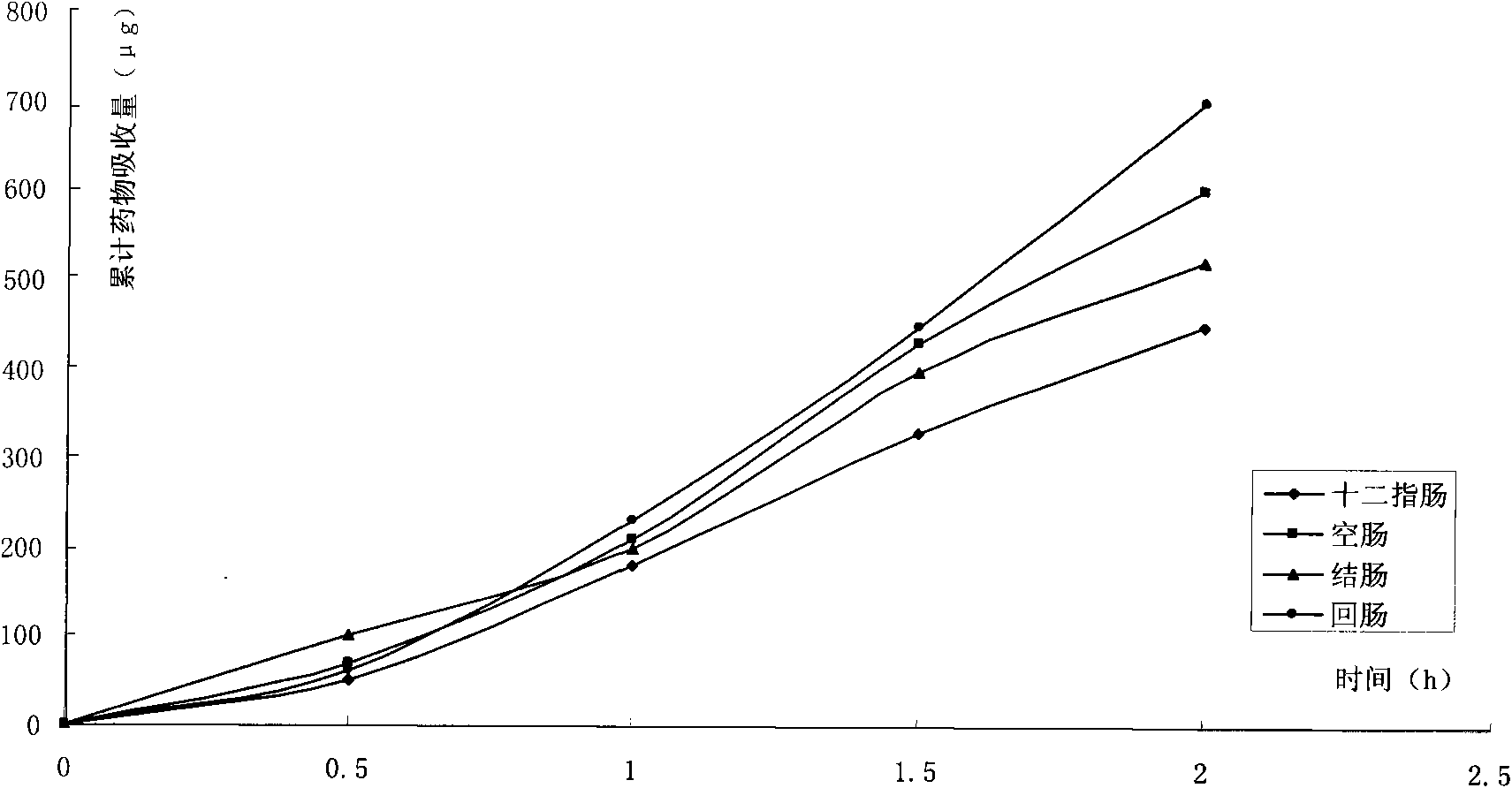
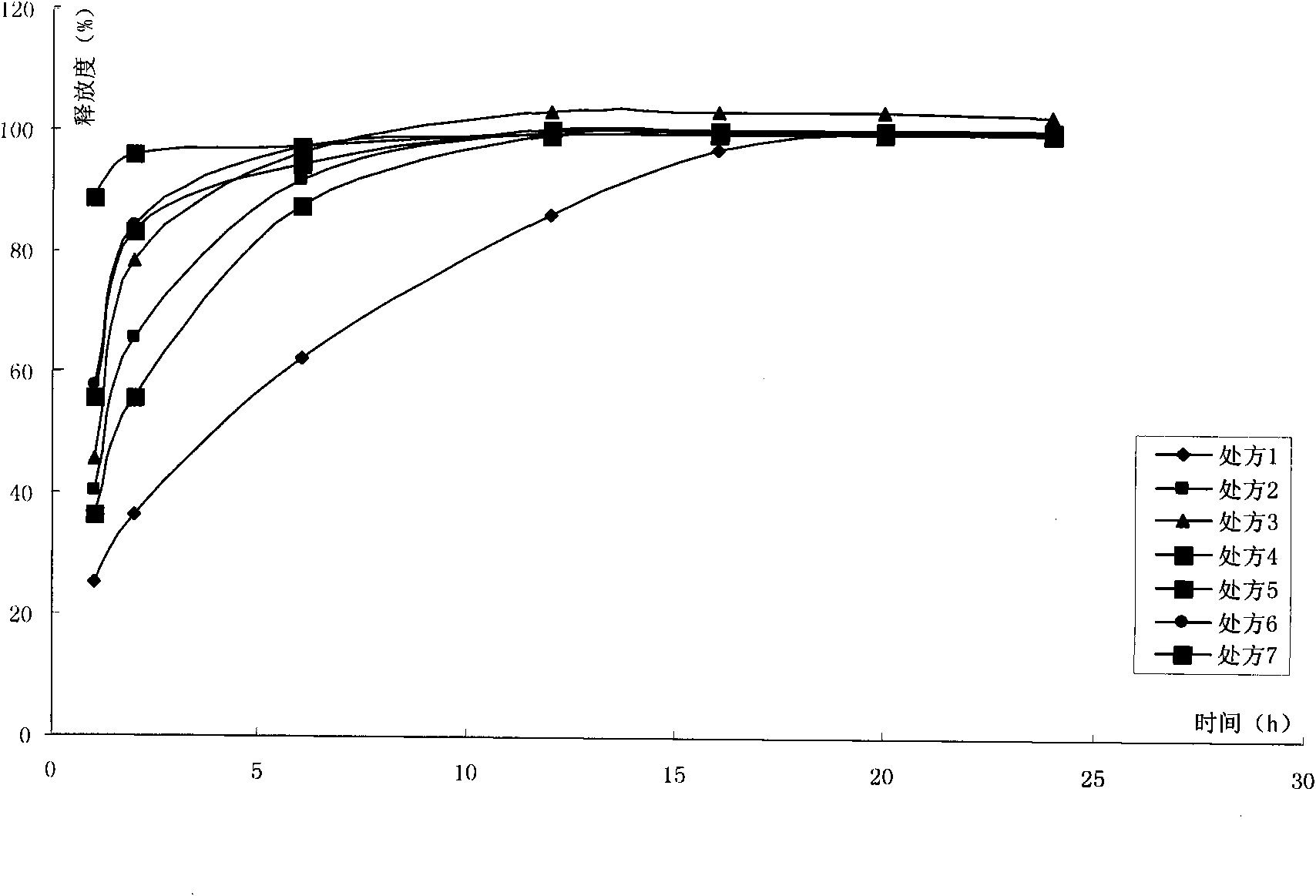
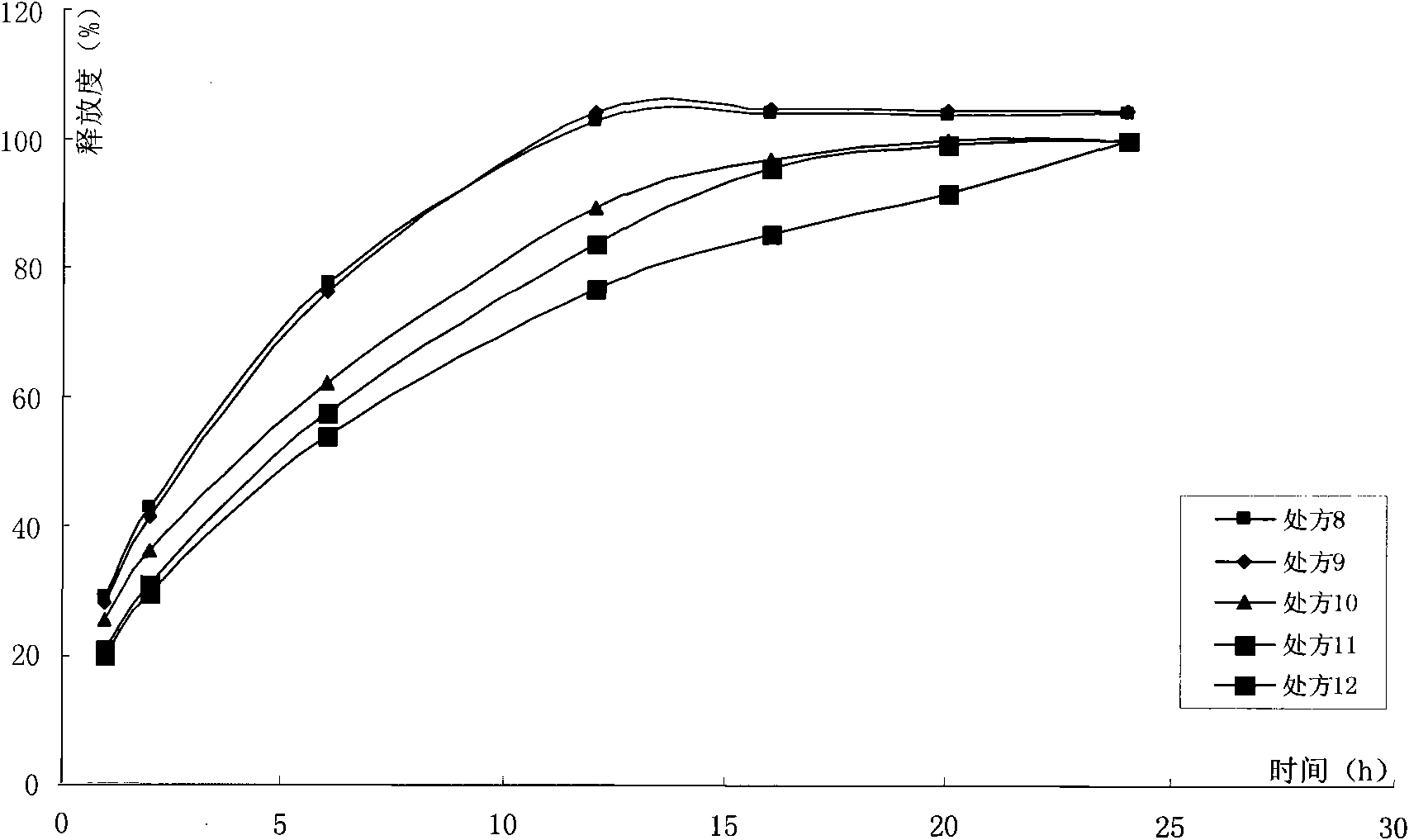
Image
Examples
Embodiment 1
[0189] Matrine 300g
[0190] Lactose 60g
[0191] 10% povidone K30 ethanol solution 60ml
[0192] Hypromellose K100M 130g
[0193] Microcrystalline Cellulose 50g
[0194] Micronized silica gel 6.0g
[0195] Magnesium stearate 3.0g made into 1000 tablets
[0196] Coating Solution Prescription:
[0197] Gastric Dissolving Film Coating Powder 10.9g
[0198] Distilled water 61.0ml
[0199] 1. Weigh matrine and lactose according to the prescription amount, mix evenly, add about 60ml of binder, stir evenly, make a suitable soft material, pass through a 18-mesh sieve to granulate;
[0200] 2. Dry the granules at 50°C, pass through a 18-mesh sieve;
[0201] 3. Determining the content of matrine in the granules and determining the tablet weight;
[0202] 4. Add the prescribed amount of hydroxypropylmethylcellulose K100M, microcrystalline cellulose, micronized silica gel, and magnesium stearate to the dry granules, and mix well;
[0203] 5. Compress the tablet with a die with ...
Embodiment 2
[0206] Matrine 300g
[0207] Lactose 50g
[0208] 10% povidone K30 ethanol solution 60ml
[0209] Hypromellose K100M 139.4g
[0210] Microcrystalline Cellulose 43.4g
[0211] Micronized silica gel 6.0g
[0212] Magnesium stearate 2.7g made into 1000 tablets
[0213] Coating Solution Prescription:
[0214] Gastric Dissolving Film Coating Powder 11.3g
[0215] Distilled water 61.0ml
[0216] 1. Weigh matrine and lactose according to the prescription amount, mix evenly, add about 60ml of binder, stir evenly, make a suitable soft material, pass through a 24-mesh sieve to granulate;
[0217] 2. Dry the granules at 60°C, pass through a 16-mesh standard sieve for granulation;
[0218] 3. Determining the content of matrine in the granules and determining the tablet weight;
[0219] 4. Add the prescribed amount of hydroxypropylmethylcellulose K100M, microcrystalline cellulose, micronized silica gel, and magnesium stearate to the dry granules, and mix well;
[0220] 5. Compres...
Embodiment 3
[0223] Matrine 300g
[0224] Lactose 59g
[0225] 10% povidone K30 ethanol solution 70ml
[0226] Hypromellose K100M 130.4g
[0227] Microcrystalline Cellulose 40g
[0228] Micronized silica gel 5.8g
[0229] Magnesium stearate 2.0g made into 1000 tablets
[0230] Coating Solution Prescription:
[0231] Gastric Dissolving Film Coating Powder 12g
[0232] Distilled water 61.0ml
[0233]1. Weigh matrine and lactose according to the prescription amount, mix evenly, add about 60ml of binder, stir evenly, make a suitable soft material, pass through a 16-mesh nylon sieve to granulate;
[0234] 2. Dry the granules at 60°C, pass through a 24-mesh standard sieve for granulation;
[0235] 3. Determining the content of matrine in the granules and determining the tablet weight;
[0236] 4. Add the prescribed amount of hydroxypropylmethylcellulose K100M, microcrystalline cellulose, micronized silica gel, and magnesium stearate to the dry granules, and mix well;
[0237] 5. Compre...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com