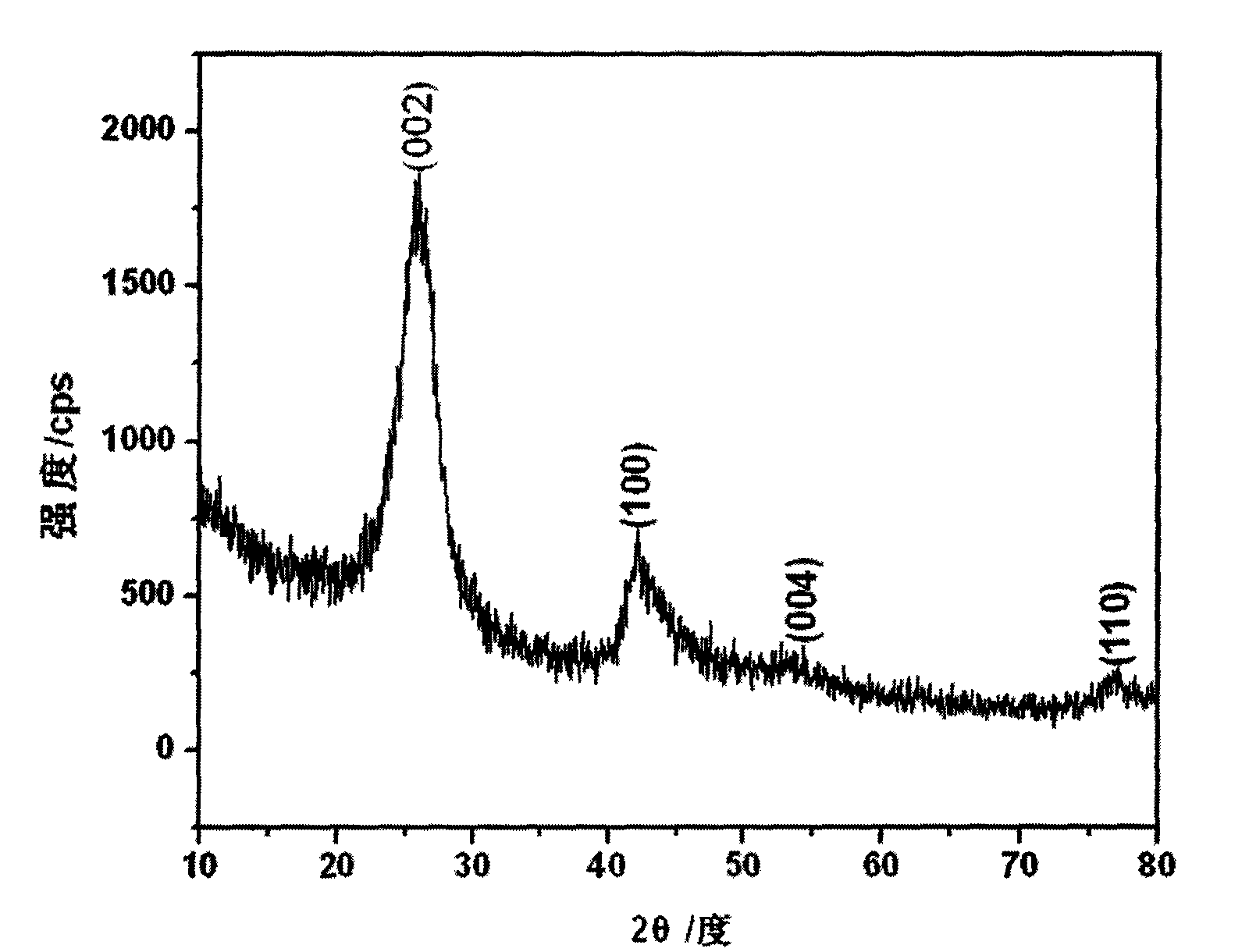
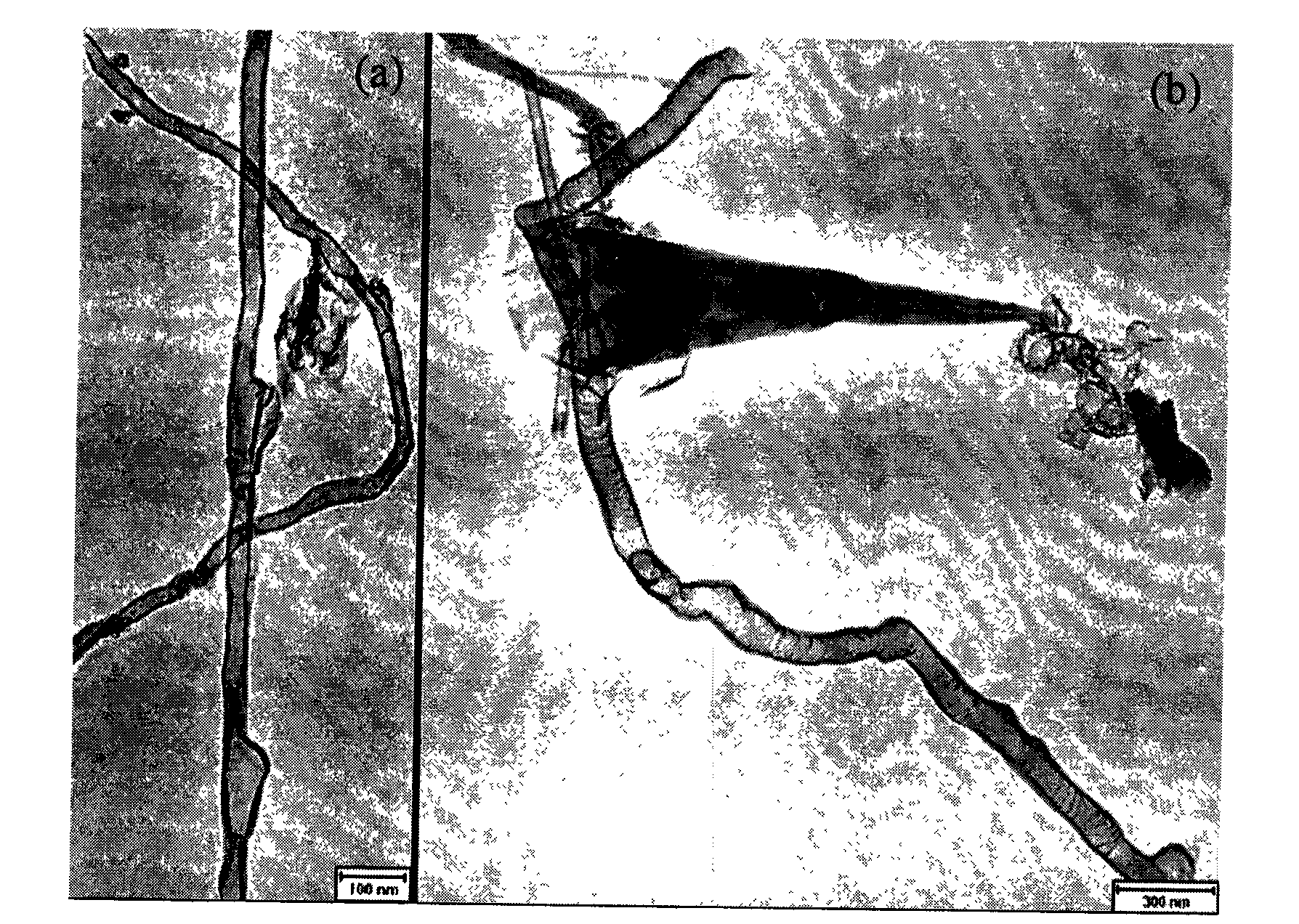
Method for preparing boron nitride nanotubes by annealing of inorganic boracic precursor
A boron nitride nanotube, boron precursor technology, applied in inorganic chemistry, chemical instruments and methods, nitrogen compounds and other directions, can solve the problems of difficulty in mass preparation, low yield, etc., and achieves low cost, simple preparation process, and reaction mild effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 Dry-blended self-propagating process
[0035] Taking calcium hexaboride (CaB 6 ) Is a boron source, cobalt oxide (Co 2 O 3 ) Introduce the preparation process of inorganic boron source precursor for catalyst The first step: ingredients. CaB 6 , Co 2 O 3 All are analytical reagents, weigh 50.03g CaB 6 , 50.25g Co 2 O 3 , Wherein the molar ratio B:Co is 1:0.21. The second step: mixing. Will weighed CaB 6 And Co 2 O 3 Add a high-speed mixer (18000 rpm) and mix for 5 minutes to make it fully uniform. The third step: molding. Pour the mixture into a steel mold, use a 500-ton press, hold the pressure for 15 minutes, shape, and take it out. The fourth step: reaction. Put the formed material into the reaction tank without sealing. Put the reaction tank in an argon-protected heating furnace, keep it at 750°C for 12 minutes, and cool it naturally. Open the tank and take out the product. The appearance is black honeycomb. The fifth step: crush. The reacted materials are adde...
Embodiment 2
[0037] Example 2 Dry powder ball milling process
[0038] Taking calcium hexaboride (CaB 6 ) Is a boron source, nickel chloride (NiCl 2 ) Introduce the preparation process of inorganic boron source precursor for the catalyst. The first step: ingredients. CaB 6 , NiCl 2 All are analytical reagents, weigh 50.04g CaB 6 , 40.09g NiCl 2 , Wherein the molar ratio B:Ni is 1:0.11. The second step: ball milling. Pour the prepared raw materials into a stainless steel ball mill tank, use stainless steel balls, adjust the parameters of the ball mill, autobiography 500 rpm, revolution 250 rpm, ball milling for 12 hours. The third step: molding. Pour the ball mill material into a steel mold, use a 500-ton press, hold the pressure for 13 minutes, shape, and take it out. The fourth step: reaction. Put the formed material into the reaction tank without sealing. Place the reaction tank in a heating furnace protected by argon, keep it at 850°C for 8 minutes, and cool it naturally. Open the tank and ...
Embodiment 3
[0040] Example 3 Liquid phase dispersion self-propagating process
[0041] With barium hexaboride (BaB 6 ) Is a source of boron, iron trioxide (Fe 2 O 3 ) Introduce the preparation process of inorganic boron source precursor for the catalyst. The first step: ingredients. BaB 6 , Fe 2 O 3 All are analytical reagents, weighing 60.03g BaB 6 , 35.09g Fe 2 O 3 , Where the molar ratio B:Fe is 1:0.24. Step 2: Disperse. Pour the weighed materials into a 1000ml beaker, add 500ml of distilled water, stir for 20 minutes, and ultrasonic for 10 minutes to make the materials evenly dispersed in the distilled water. The third step: suction filtration. The mixed liquid is filtered through a circulating water vacuum pump. The fourth step: drying. The obtained filter cake was dried in a vacuum drying oven at 80°C for 8 hours. The fifth step: crush. Add the dried filter cake to a high-speed mixer (18000 rpm) and mix for 5 minutes. The sixth step: molding. Pour the mixture into a steel mold, use a 50...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com