Method for removing iron and enriching nickel cobalt through precipitation of laterite type nickel ores
A technology of laterite nickel ore and nickel-cobalt, applied in the direction of improving process efficiency, etc., can solve the problems of difficult separation and utilization of base metals, high energy consumption of hydrochloric acid regeneration, etc., to solve the difficult separation of base metal elements, improve recovery rate, reduce The effect of production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
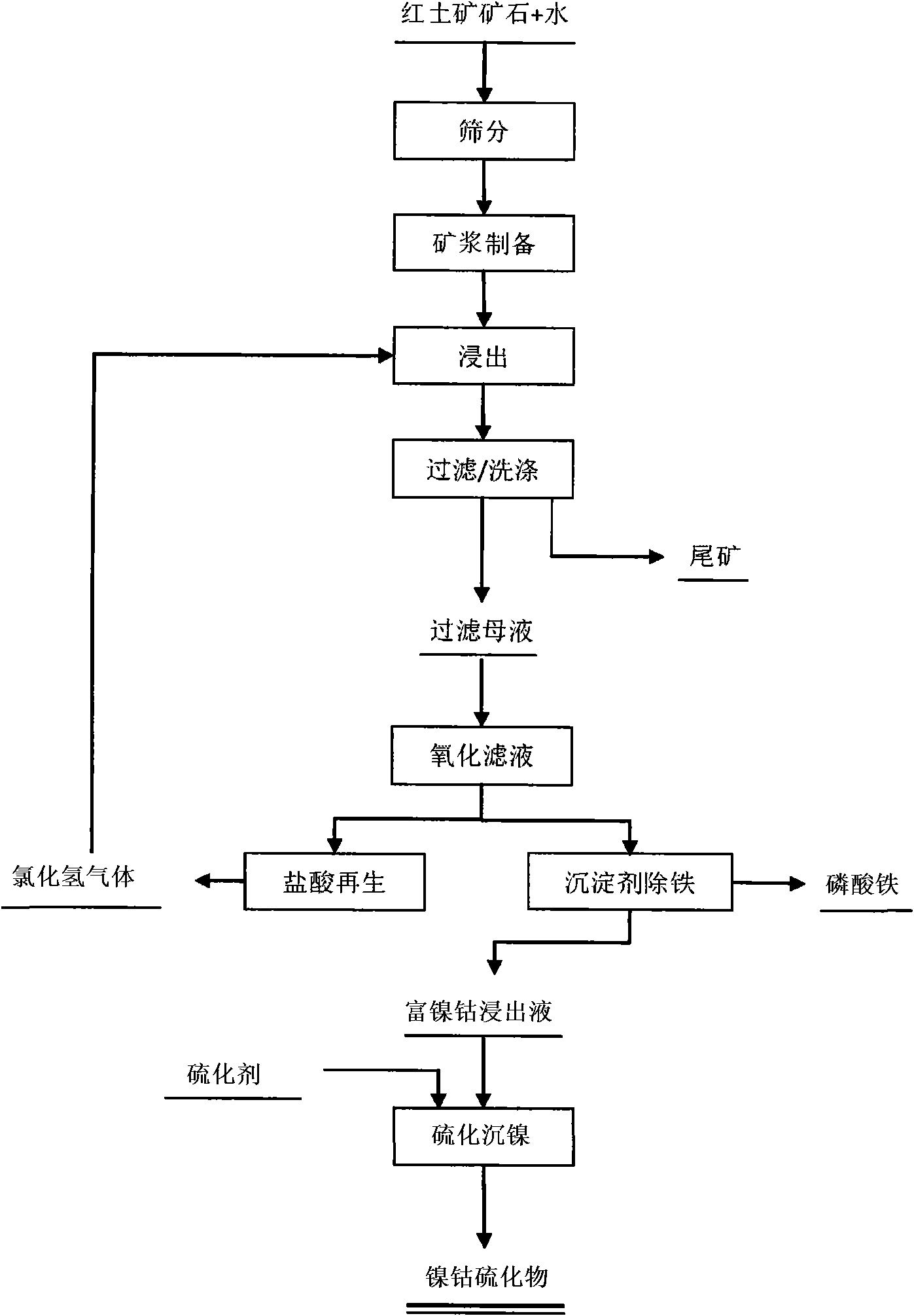
[0022] The laterite nickel ore is ball-milled and passed through a 50-mesh sieve, and 500 grams of -50-mesh ore sample is leached with hydrochloric acid, so that the concentration of Fe in the leachate is 0.1mol / L, and a sufficient amount of sodium peroxide solution (1mol / L ), then add phosphoric acid (1mol / L) equimolar to Fe, adjust the pH=2.5±0.1 with sodium hydroxide solution (0.5mol / L), react in a stirred reactor at 50°C for 5min, and wash the resulting precipitate, Filtrate, and recover the regenerated hydrochloric acid to the leaching process for recycling. Hydrogen sulfide was added to the filtrate, reacted for 1 hour, and a nickel-cobalt-rich precipitate of sulfide was obtained after solid-liquid separation. After analysis, from the ore to the completion of sulfide precipitation, the comprehensive recovery rate of nickel and cobalt: Ni 93.32%; Co80.97%.
Embodiment 2
[0024] The laterite nickel ore is ball-milled and passed through a 50-mesh sieve, and 500 grams of -50-mesh ore samples are taken and leached with hydrochloric acid, so that the concentration of Fe in the leach solution is 1mol / L, and a sufficient amount of hydrogen peroxide solution (0.01mol / L) is added to the solution, Then add triammonium phosphate (0.01mol / L) equimolar to Fe, adjust pH=2.0±0.1 with sodium hydroxide solution (6mol / L), react in a stirred reactor at 40°C for 5min, and wash the resulting precipitate, Filtrate, and recover the regenerated hydrochloric acid to the leaching process for recycling. Hydrogen persulfide was added to the filtrate, reacted for 24 hours, and a nickel-cobalt-rich precipitate of sulfide was obtained after solid-liquid separation. After analysis, from the ore to the completion of sulfide precipitation, the comprehensive recovery rate of nickel and cobalt is: Ni 90.83%; Co 78.51%.
Embodiment 3
[0026] The laterite nickel ore is ball-milled and crossed a 50-mesh sieve, and 500 grams of -50-mesh ore sample is leached with hydrochloric acid, so that the concentration of Fe in the leachate is 0.01mol / L, and a sufficient amount of potassium permanganate solution (9mol / L L), then add ammonium dihydrogen phosphate (9mol / L) equimolar with Fe, adjust pH=0.1 ± 0.1 with sodium hydroxide solution (0.01mol / L), react 5min in the stirring reactor of 30 ℃, will The obtained precipitate is washed, filtered, and the regenerated hydrochloric acid is recovered to the leaching process for recycling. Add sodium hydrosulfide to the filtrate, react for 1 minute, and obtain nickel-cobalt-enriched sulfide precipitate after solid-liquid separation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com