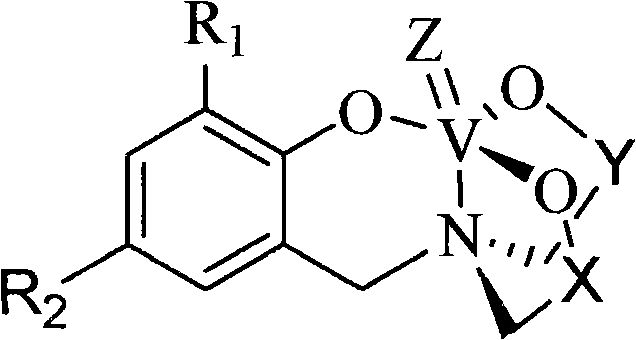
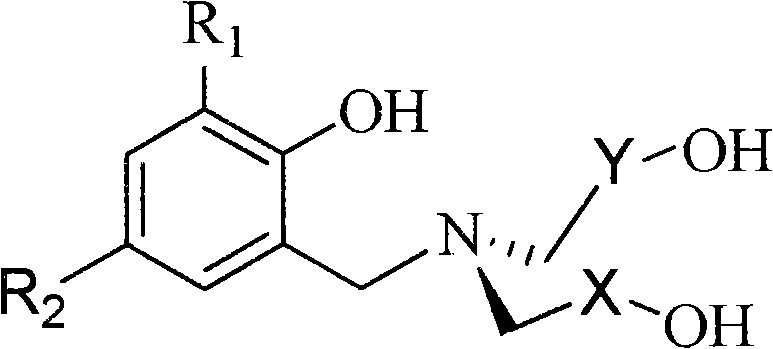
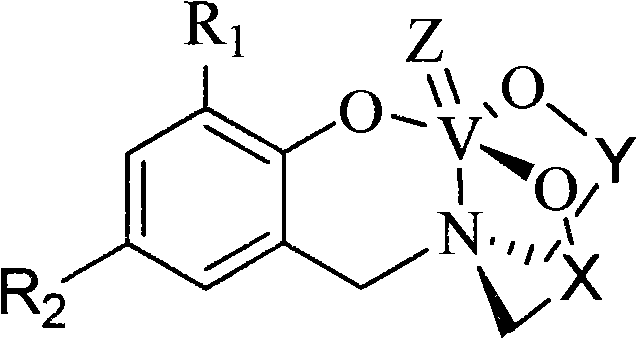
Polyhydroxy pentavalent vanadium olefin polymerization catalyst, preparation method and application
A polyhydroxy pentavalent vanadium catalyst and a technology for olefin polymerization, which are applied in the polyhydroxy pentavalent vanadium olefin polymerization catalyst, preparation method and application field, and can solve the problems of weak ability to catalyze olefin copolymerization, poor high temperature resistance and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Under an argon atmosphere, a solution of 0.255 g (1.0 mmol) of polyhydroxy ligand 1a in 20 ml of methylene chloride was transferred to 0.244 g (1.0 mmol) of VO(O n Pr) 3 20ml of dichloromethane solution, continue to stir at room temperature for 12h. The solution was concentrated to 10ml, and 20ml of anhydrous n-hexane was added to precipitate a black-red crystalline compound to obtain 0.239g (74.9%) of complex 2a. 1 H NMR (CDCl 3 , 25°C): δ6.65(s, 1H, Ar-H), 6.52(s, 1H, Ar-H), 4.71(t, J=3.0Hz, 4H, OCH 2 ), 3.87 (s, 3H, OCH 3 ), 3.83 (s, 2H, ArCH 2 ), 2.91(t, J=6.0Hz, 4H, NCH 2 ), 2.28(s, 3H, ArCH 3 ). Mass spectrometry, the molecular ion peak m / e is 319. Elemental analysis found values: C, 48.98%; H, 5.69%; N, 4.37%; theoretical values: C, 48.91%; H, 5.68%; N, 4.39%.
[0033] The structural formula of the polyhydroxy ligand 1a is as follows:
[0034]
[0035] The structural formula of the complex 2a is as follows:
[0036]
Embodiment 2
[0037] Example 2 Under an argon atmosphere, a solution of 0.283 g (1.0 mmol) of polyhydroxy ligand 1b in 20 ml of methylene chloride was transferred to 0.244 g (1.0 mmol) of VO(O n Pr) 3 20ml of dichloromethane solution, continue to stir at room temperature for 12h. The solution was concentrated to 10ml, and 20ml of anhydrous n-hexane was added to precipitate a black-red crystalline compound to obtain 0.265g (76.5%) of complex 2b: 1 H NMR (CDCl 3 , 25°C): δ6.68(s, 1H, Ar-H), 6.65(s, 1H, Ar-H), 6.53(s, 1H, Ar-H), 6.49(s, 1H, Ar-H) , 5.03(sept, J=4.2Hz, 1H, CH), 4.64(sept, J=4.2Hz, 1H, CH), 3.88(s, 3H, OCH 3 ), 3.74 (penta, J=55.8Hz, 2H, ArCH 2 ), 2.71 (d, J=12.0Hz, 2H, NCH 2 ), 2.51 (d, J=12.0Hz, 2H, NCH 2 ), 2.28(s, 3H, ArCH 3 ), 1.22 (m, 6H, CHCH 3 ). Mass spectrometry, the molecular ion peak m / e is 347. Elemental analysis found values: C, 51.94%; H, 6.95%; N, 4.00%; Theoretical values: C, 51.88%; H, 6.93%; N, 4.03%.
[0038] The structural formula of the polyhydrox...
Embodiment 3
[0042] Example 3 Under an argon atmosphere, a solution of 0.361 g (1.0 mmol) of polyhydroxy ligand 1c in 20 ml of methylene chloride was transferred to 0.244 g (1.0 mmol) of VO(O n Pr) 3 20ml of dichloromethane solution, continue to stir at room temperature for 6h. The solution was concentrated to 10ml, and 20ml of anhydrous n-hexane was added to precipitate a black-red crystalline compound to obtain 0.354g (83.3%) of complex 2c: 1 H NMR (CDCl 3 , 25°C): δ6.60 (s, 2H, Ar-H), 6.49 (s, 2H, Ar-H), 4.82 (br, 2H, OCH 2 ), 3.85(s, 6H, OCH 3 ), 3.45 (br, 4H, ArCH 2 ), 2.72 (br, 2H, NCH 2 ), 2.26(s, 6H, ArCH 3 ). Mass spectrometry, the molecular ion peak m / e is 425. Elemental analysis found values: C, 56.41%; H, 5.66%; N, 3.31%; theoretical values: C, 56.47%; H, 5.69%; N, 3.29%.
[0043] The structural formula of the polyhydroxy ligand 1c is as follows:
[0044]
[0045] The structural formula of the complex 2c is as follows:
[0046]
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com