Jervine steroid alkaloid derivatives and preparation and application thereof
A technology of steroidal alkaloids and derivatives, which is applied in the field of medicine and can solve problems such as high toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
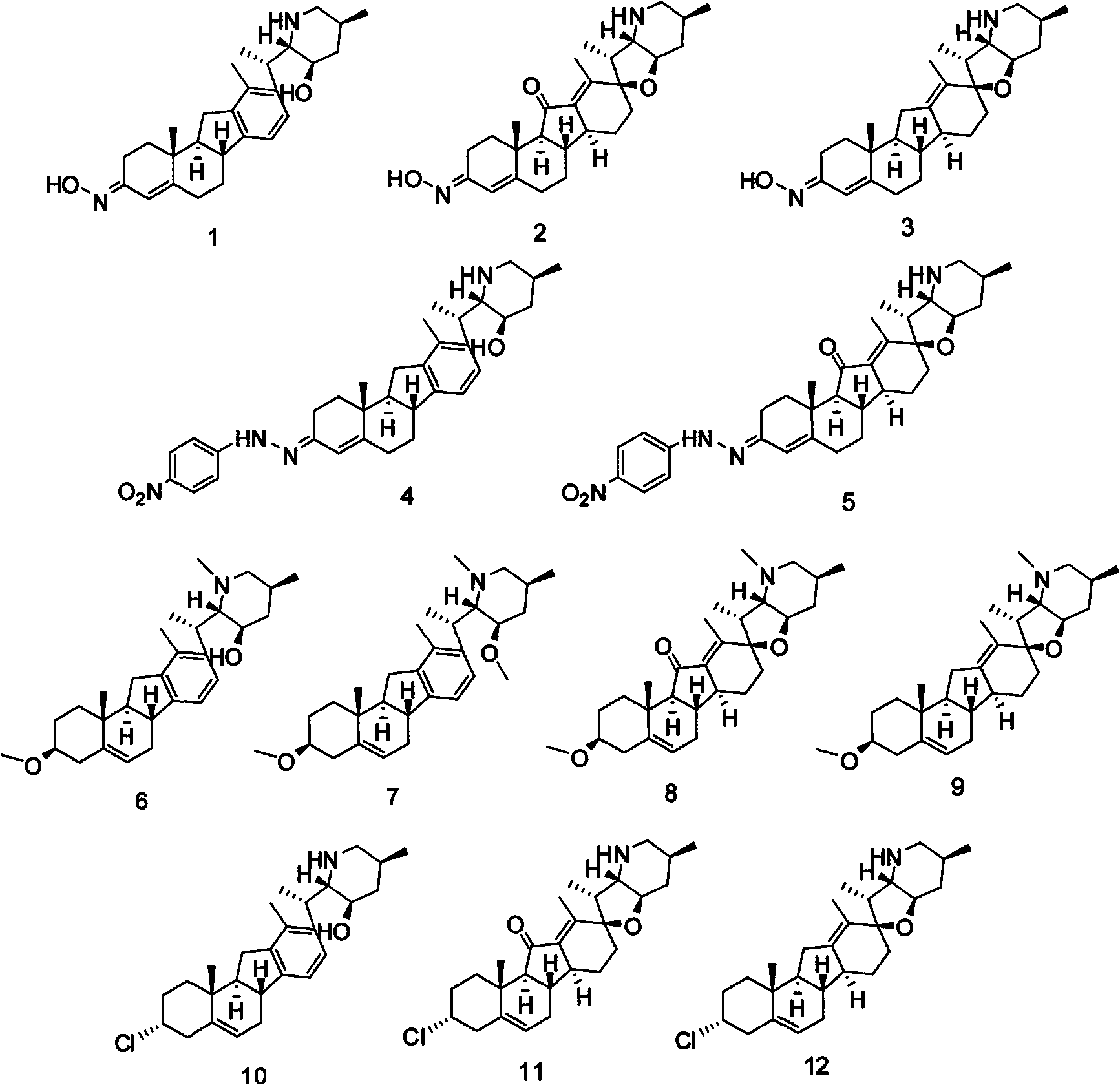
[0031] Put 25mg (0.06mmol) 3-carbonyl-Δ 4 -Veratramine was dissolved in 15 mL of methanol, and 18 mg (0.25 mmol) of hydroxylamine hydrochloride was added. Reflux for 4h. After the reaction was completed, it was concentrated to 2 mL and placed in a refrigerator. 111 mg of white crystals were precipitated with a yield of 42.4%.
[0032] Compound 1:
[0033] White powder, ESI-MS: m / z 423.3[M+H] + , 1H-NMR (400MHz, CD 3 OD, J / Hz) δ: 7.11 (d, 1H, J=13, H-16), 6.97 (d, 1H, J=13, H-15), 5.85 (s, 1H, H-4), 3.53 (m, 1H, H-20), 3.16 (m, 1H, H-23), 3.05 (t, 1H, J=7, H-6a), 2.98 (t, 1H, H-8), 2.85 (dd , 1H, J=4, H-26a), 2.77(dd, 1H, H-11), 2.48(m, 1H, H-23), 2.31(s, 3H, H-18), 2.08(t, 1H , J=4, H-26b), 2.00 (brs, 1H, H-24a), 1.50 (d, 3H, H-21), 1.18 (s, 3H, H-19), 1.05 (dd, 1H, H -24b) 0.83 (d, 3H, H-27); 13C-NMR (400MHz, CD 3 OD)δ: 157.5(C-5), 154.9(C-3), 144.7(C-12), 143.7(C-14), 141.2(C-17), 133.9(C-13), 126.5(C- 16), 120.5(C-15), 119.7(C-4), 71.7(C-23), 67.9(C-22), 61.5(C-9), 54.5(C-26), ...
Embodiment 2
[0040] Dissolve 100mg (0.24mmol) of veratramine in 10mL of acetone and add 0.3gK 2 CO 3 (2.4mmol) and 0.5mL (5.2mmol) of dimethyl sulfate (DMS), react at 25°C overnight. After the solvent was evaporated, 30 mL of water was added, and the mixture was extracted with 3×30 mL of ethyl acetate. The organic layer was washed with 30 mL of saturated brine and washed with anhydrous Na 2 SO 4 dry. The solvent was evaporated to obtain 102 mg of the crude product, which was separated by silica gel column to obtain compounds 6 (31 mg) and 7 (8 mg).
[0041] Compound 6: White powder, ESI-MS: m / z 438.3 [M+H]+ ;
[0042] Compound 7: White powder, ESI-MS: m / z 452.4 [M+H] + ;
[0043] The same experimental operation, except that the reactants were changed to fenfenamine and cyclopamine, respectively, and 8 and 9 were obtained correspondingly.
[0044] Compound 8: White powder, ESI-MS: m / z 454.3 [M+H] + ;
[0045] Compound 9: white powder, ESI-MS: m / z 440.4 [M+H] + ;
Embodiment 3
[0047] Dissolve 41mg (0.1mmol) veratramine in 8mL pyridine, add SOCl dropwise after cooling in ice water 2 0.2mL (2.8mmol), gradually increase the temperature to 25°C, and react overnight. TLC indicated that the reaction was over, 50mL ethyl acetate was added, washed with 3×30mL water, the organic layer was washed with 30mL saturated brine, and anhydrous Na 2 SO 4 dry. The solvent was evaporated to obtain 40 mg of crude product, which was separated by silica gel column to obtain compound 10 (20 mg).
[0048] The same experimental operation, except that the reactants were changed to fenfenamine and cyclopamine respectively, and 11 and 12 were obtained correspondingly.
[0049] Compound 10: white powder, ESI-MS: m / z 428.3 [M+H] + ;
[0050] Compound 11: white powder, ESI-MS: m / z 444.3[M+H] + ;
[0051] Compound 12: white powder, ESI-MS: m / z 430.4 [M+H] + ;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com