New medical use of cucurbitacin in treating cancer
A technology of cucurbitacin, which is applied in the field of medicine, can solve problems such as limiting the scope of application, increasing risks, and patients with leukopenia, and achieves the effect of increasing the number of white blood cells, improving the quality of life, and good therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1: Cucurbitacin B (CuB, the purity determined by HPLC area normalization method is greater than 99%) in vitro anti-human pancreatic cancer cell research
[0020] In vitro test (MTT)
[0021] method:
[0022] 1. Human pancreatic cancer cells BxPC-3 were cultured with RPMI 1640 medium containing 10% fetal bovine serum at 37°C and 5% CO 2 In the incubator, subculture once every 3-4 days. Cells in the logarithmic growth phase were selected for the experiment.
[0023] 2. The adherent cells were digested with 0.25% trypsin (containing EDTA 1mM) and suspended in the culture medium to adjust the cell concentration to 5×10 4 / mL, inoculate 100 μL per well on a 96-well plate, with 3 replicate wells in each well. Only culture solution was added to blank control wells.
[0024] 3. Cultivate the cells overnight. After the cells adhere to the wall, add different concentrations of drugs (0.5 μL) into groups, and the other group is the control group (without adding drugs)...
Embodiment 2
[0030] Example 2: Anti-human pancreatic cancer pharmacodynamics study of cucurbitacin B (CuB, the purity determined by HPLC area normalization method is greater than 99%) in vivo
[0031] method:
[0032] 1. Human pancreatic cancer cells BxPC-3 were cultured with RPMI1640 medium containing 10% fetal bovine serum in 37, 5% CO 2 In the incubator, subculture once every 3-4 days. Cells in the logarithmic growth phase were selected for the experiment.
[0033] 2. Digest the adherent cells with 0.25% trypsin (containing EDTA 1mM) and suspend them in normal saline to adjust the cell concentration to 2.5×10 7 / mL.
[0034] 3. 36 18-20 g nude mice (BALB / C, 5-6 weeks, female, purchased from the Institute of Experimental Animals, Chinese Academy of Medical Sciences, Chinese Union Medical University) were inoculated with 5×10 cells in the right underarm 6 / only, when the tumor grows to be larger than 100mm 3 At the same time, they were randomly divided into 6 groups, which were nega...
Embodiment 3
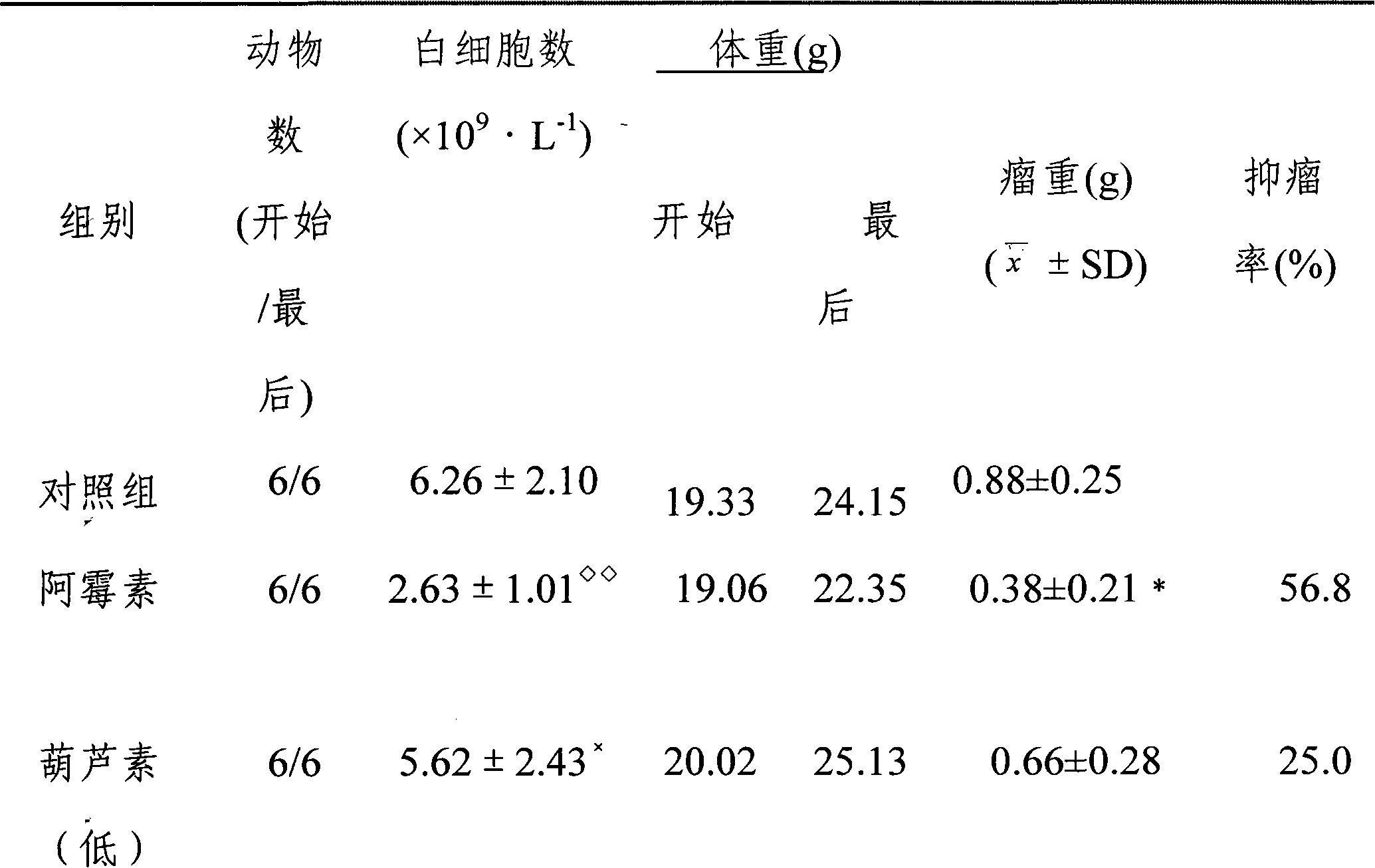
[0044] Example 3: Pharmacodynamic study of cucurbitacin administered orally and gavage against human pancreatic cancer, refer to the method in "Example 2"
[0045] 24 nude mice of 18-20 g, inoculated with 5×10 cells in the right armpit 6 / only, when the tumor grows to be larger than 100mm 3 At the same time, they were randomly divided into 4 groups, which were negative control group (MCT, 0.2ml / day), cucurbitacin group (cucurbitacin was dissolved with MCT, and the dosage was calculated according to the weight of cucurbitacin, administered by intragastric administration, 150 μg / day). kg·day, 300 μg / kg·day, 600 μg / kg·day), for 14 consecutive days. The animals were sacrificed within 24 hours after the last administration, and the tumors were weighed to calculate the tumor inhibition rate. The results are shown in Table 2.
[0046] The antitumor effect of table 2 cucurbitacin intragastric administration ( )
[0047]
[0048] Cucurbitacin
(Low)
Cucur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com