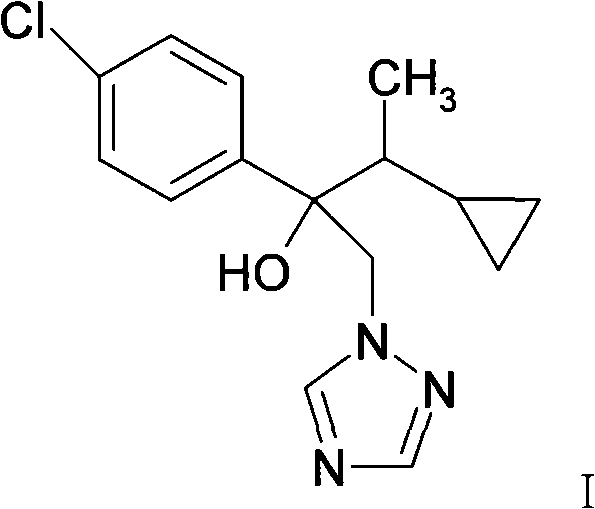
Preparation process for cyproconazole
A preparation process, the technology of cyclaconazole, which is applied in the field of preparation of the pesticide cyclaconazole, can solve the problems of time-consuming, laborious, uneconomical, and environmental pollution, and achieve the effects of simple and safe operation, less environmental pollution, and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Add 250 g of sulfide into a 500 ml three-necked flask, add 30 g of tert-butanol dropwise, then stir evenly, keep the internal temperature below 35 ℃, add 50 g of dimethyl sulfate dropwise, after the addition, stir for 4 hours, then add 1- 75 grams of (4-chlorophenyl)-2-cyclopropyl-1-acetone, 50 grams of potassium hydroxide, and stirring at about 40°C for 20 hours, sampling and analysis of raw materials should be less than 1%, the reaction solution is poured into cold water, Stir and dissolve completely, stand still for layering, wash the organic layer with an appropriate amount of water to neutrality, firstly remove the solvent under normal pressure, and then remove the low-boiling substances under reduced pressure to obtain 2-(4-chlorophenyl)-2-(1-ring) Propyl ethyl) ethylene oxide is about 82 grams, with a content of 90% (quantitative analysis by gas chromatography, external standard method).
[0029] 82 grams of the above 2-(4-chlorophenyl)-2-(1-cyclopropylethyl)oxirane ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com