Double labelling Nano-Au probe and preparation method
A nano-gold probe and nano-gold technology, applied in the double-labeled nano-gold probe and its preparation, the application field of the nano-gold probe in the detection of ultra-trace antigens, which can solve the problems of inability to use immunoassays and difficulties in practical promotion and application, etc. problems, to achieve good clinical application value, improve detection sensitivity, and easy operation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Trisodium citrate (Shanghai Sinopharm) reduced HAuCl 4 Preparation of colloidal gold. 100ml of 0.01% HAuCl 4 Solution is heated to boiling; Under high-speed stirring condition (1200rpm), add 5ml 1% trisodium citrate (Na 3 C 6 h 5 o 7 2H 2 0), continue heating and stirring for 30min, cool naturally, and return to the original volume with ultrapure water. Filter through a 0.22 μm filter membrane and store in the dark at 4°C for later use.
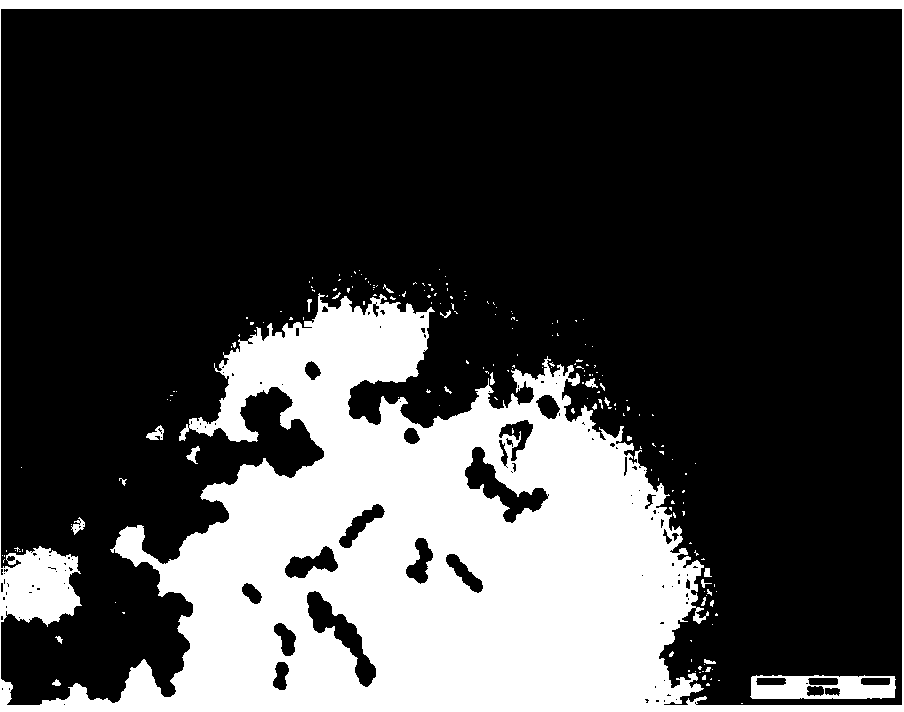
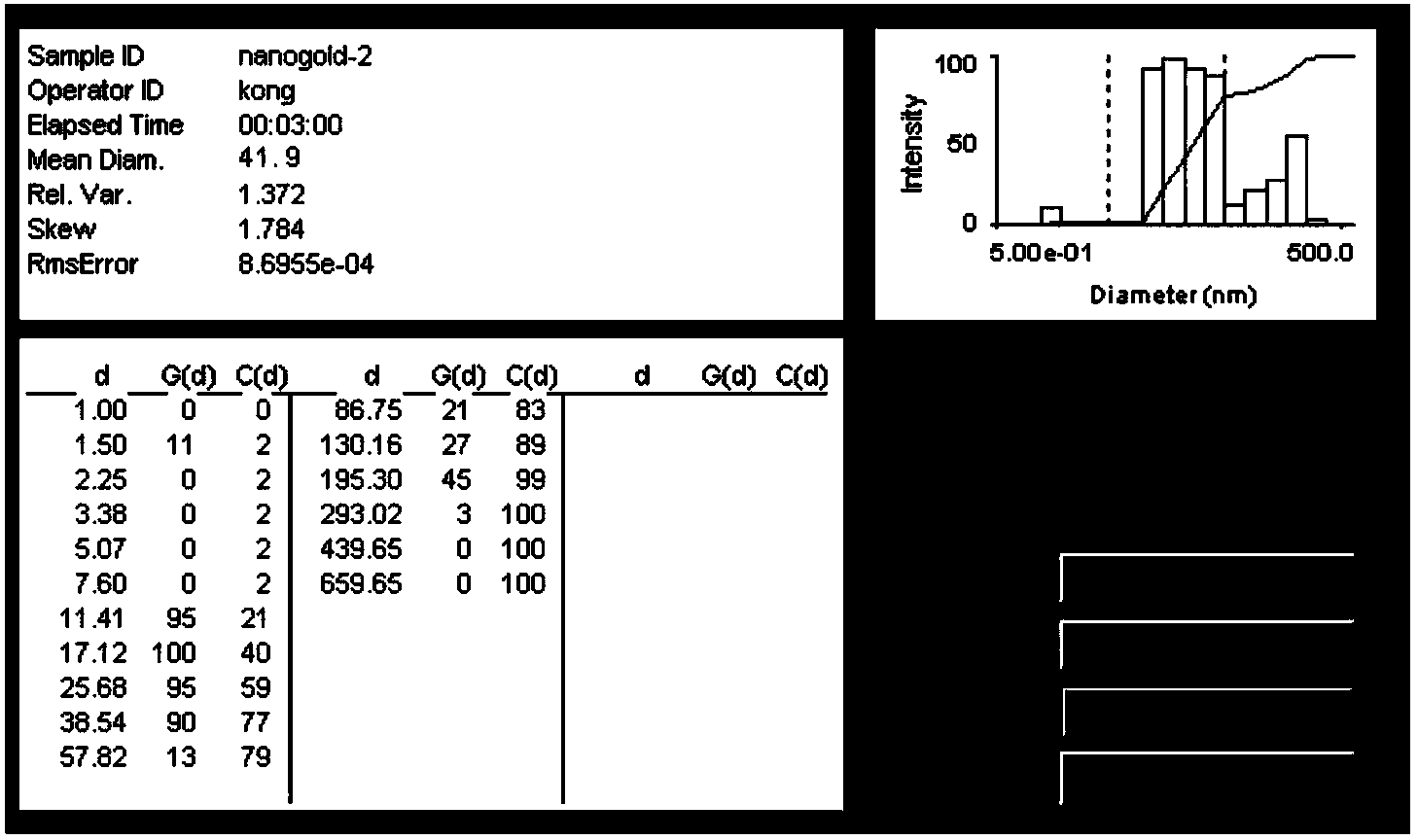
[0061] Such as figure 1 As shown, the resulting nano-gold was observed with a transmission electron microscope (TEM) to observe the morphology of the particles, and its size and particle size distribution were measured with a laser scattering instrument. concentration of gold nanoparticles.
[0062] Experimental results The obtained nano-gold has the largest absorption peak at 518nm, uniform particle size, good dispersion, the main distribution range is 10±3nm, and the approximate concentration of gold colloid is 3.7nM.
[0...
Embodiment 2
[0065]Get 100ml 0.01% HAuCl solution and heat it to boiling; add 2.5-4.5ml 1% trisodium citrate (Na 3 C 6 h 5 o 7 2H 2 0), continue heating and stirring for 30min, cool naturally, and return to the original volume with ultrapure water. Filter through a 0.22 μm filter membrane and store in the dark at 4°C for later use.
[0066] Experimental results The particle size of the obtained nano gold particles has a maximum absorption peak at about 520-534nm, and the particle size is about 15-30nm.
[0067] 2. Determination of the optimum pH and optimum stable amount of antibody-bound gold nanoparticles
Embodiment 3
[0069] Adopt the nano-gold prepared in Example 1 of optimized preparation conditions, get several 1.5ml small test tubes, add 1ml nano-gold solution respectively; 2 CO 3 Adjust the pH to 3, 4, 5, 6, 7, 8, 9, and 10 in turn; take a 96-well plate, add 100 μl of the above colloidal gold into the wells according to the pH from low to high, and repeat; Add 2μl of antigen or antibody with a concentration of 1mg / ml to the wells, mix, and place at room temperature for 10-15min; then add a certain amount of 1.2M NaCl solution to each well, mix, and place at room temperature for 10min; Observe the color change of colloidal gold , record the lowest pH (X) that keeps red; and repeat the above steps, the pH gradient is X-0.6; X-0.3; X; X+0.3; X+0.6; X+1. Observe the color change of the colloidal gold until it is placed at room temperature for 2 hours, and record the lowest pH that remains red.
[0070] The experimental results show that the optimal pH for the combination of the gold nano...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com