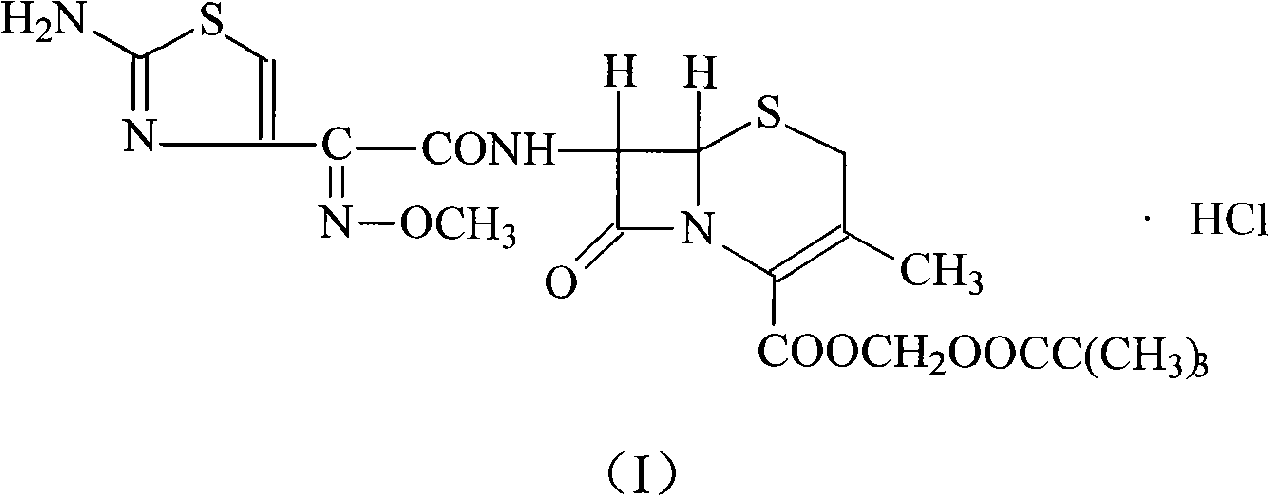
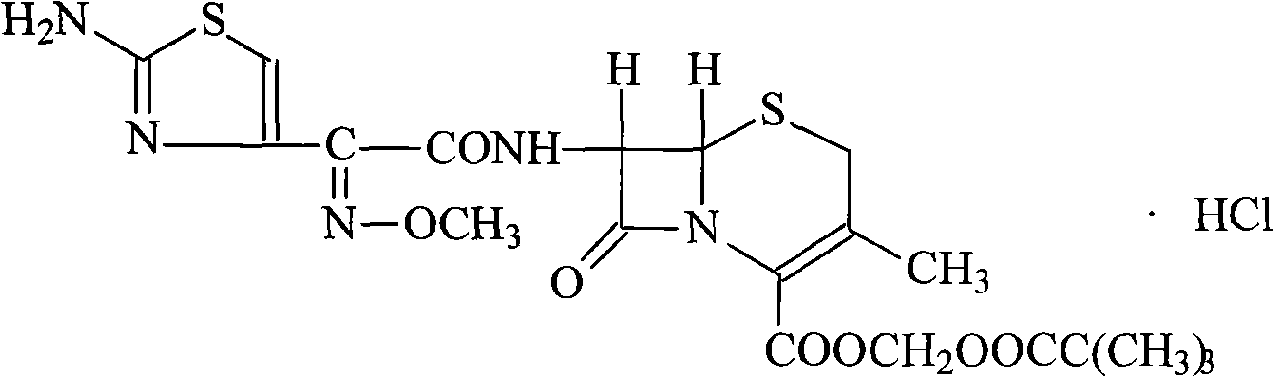
Cefetamet pivoxil hydrochloride compound and preparation method thereof
A technology for ceftazime pivoxil hydrochloride and a compound, which is applied in the refining field of ceftazidime pivoxil hydrochloride compounds, can solve the problems of difficulty in preparing preparations, unattainable preparations, low purity of cefetamet pivoxil hydrochloride, etc., and achieves low cost , improve the quality, the effect of convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] (1) Dissolve 100 g of ceftazidime pivoxil hydrochloride crude product in 600 ml of methanol, add 230 ml of 2 mol / L sodium hydroxide solution, stir and react at room temperature for 60 minutes, hydrolyze to separate crystals, filter, wash with water, and dry under reduced pressure at 50°C , to obtain 95.4 g of cefetamet sodium salt, with a yield of 95.4%;
[0021] (2) Dissolve cefetamet sodium salt in 800ml of water, add 8g of activated carbon for adsorption for 30 minutes, filter and decarburize, then add 88.3g of iodomethyl pivalate and 300ml of N,N-dimethylformamide, and stir at room temperature for reaction After 60 minutes, add 600ml of ethyl acetate and 100ml of water, stir and extract, separate layers, wash the organic phase with 400ml of water and saturated sodium carbonate solution, then dry with anhydrous sodium sulfate, filter, concentrate under reduced pressure, and evaporate the organic solvent , and dried under reduced pressure at 50° C. to obtain 89.5 g of...
Embodiment 2
[0024] (1) Dissolve 100 g of ceftazidime pivoxil hydrochloride crude product in 800 ml of ethanol, add 250 ml of 2 mol / L potassium hydroxide solution, stir and react at room temperature for 60 minutes, hydrolyze to separate crystals, filter, wash with water, and dry under reduced pressure at 50°C , to obtain 96.7g of cefetamet potassium salt, with a yield of 96.7%;
[0025] (2) Dissolve cefetamet potassium salt in 1000ml of water, add 10g of activated carbon for adsorption for 30 minutes, filter for decarburization, then add 102.2g of iodomethyl pivalate and 500ml of N,N-dimethylsulfoxide, and stir at room temperature for reaction After 60 minutes, add 800ml of ethyl acetate and 200ml of water, stir and extract, separate layers, wash the organic phase with 600ml of water and saturated sodium carbonate solution, then dry with anhydrous magnesium sulfate, filter, concentrate under reduced pressure, and evaporate the organic solvent , dried under reduced pressure at 50° C. to obt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com