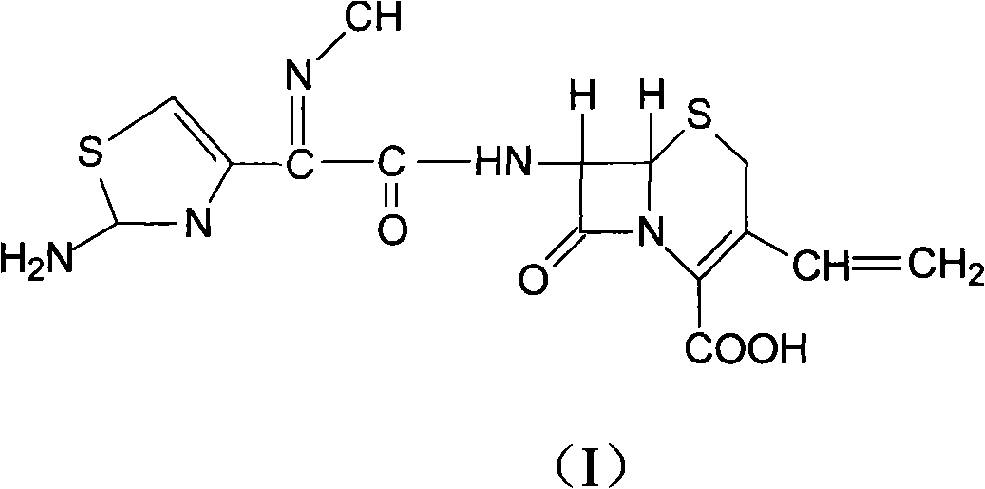
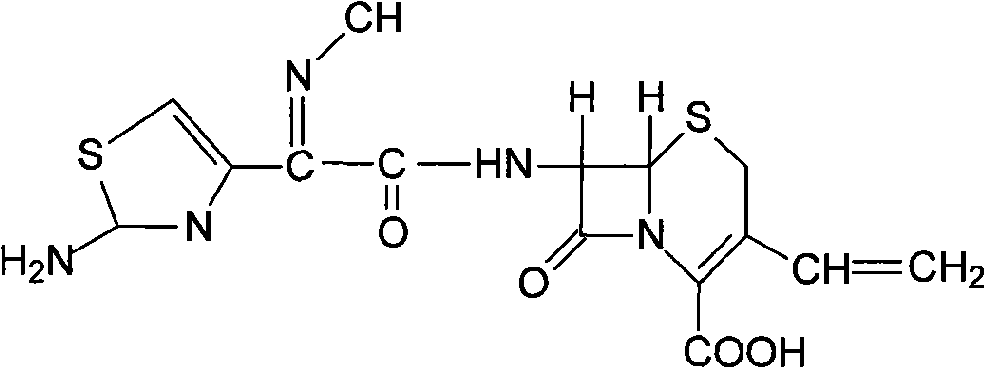
Cefdinir compound and preparation method thereof
A technology for cefdinir and compounds, which is applied in the field of refining cefdinir compounds, can solve the problems of poor stability, low purity and low cefdinir purity, and achieves the effects of low cost, simple process and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Take 100 g of cefdinir crude product, add 2 mol / L sodium hydroxide solution, fully stir until clarified to obtain cefdinir sodium solution, add 5 g of activated carbon to absorb at room temperature for 30 minutes, filter for decarburization, add 10% sodium dihydrogen phosphate solution to the filtrate , stirred and reacted, crystals were precipitated, filtered, washed with water, and dried under reduced pressure at 50° C. to obtain 92.2 g of cefdinir refined product, with a yield of 92.2%, and a purity of 99.7% by HPLC.
[0017] Structural confirmation: Elemental analysis theoretical value C: 42.5%, H: 3.3%, N: 17.7%, O: 20.2%, S: 16.2%; experimental value C: 42.4%, H: 3.4%, N: 17.5%, O : 20.3%, S: 16.3%.
[0018] H-NMR (δ, DMSO-d 6 ): 3.56, 3.87 (2H, Abq, C-2), 5.21 (1H, d, C-6), 5.32 (1H, d, -CH=CH 2 ), 5.63 (1H, d, -CH=CH 2 ), 5.79-5.84 (1H, m, C-7), 6.68 (1H, s, aminothiazole ring-H), 6.89-6.98 (1H, m, -CH=CH 2 ), 7.15 (2H, brds, -NH 2 ), 9.79 (1H, d, -NH-).
Embodiment 2
[0020] Take 100 g of cefdinir crude product, dissolve it in 500 ml of acetonitrile, add 5 mol / L of sodium acetate solution, stir well until clarification, to obtain cefdinir sodium solution, add 8 g of activated carbon to absorb at room temperature for 20 minutes, filter for decarburization, add 1 mol / L of filtrate L hydrochloric acid solution, stirred and reacted, precipitated crystals, filtered, washed with water, and dried under reduced pressure at 40°C to obtain 90.8g of cefdinir refined product, with a yield of 90.8%, and a purity of 99.6% by HPLC.
[0021] Structural confirmation: Elemental analysis theoretical value C: 42.5%, H: 3.3%, N: 17.7%, O: 20.2%, S: 16.2%; experimental value C: 42.6%, H: 3.3%, N: 17.6%, O : 20.4%, S: 16.1%.
[0022] H-NMR (δ, DMSO-d 6 ): 3.57, 3.87 (2H, Abq, C-2), 5.22 (1H, d, C-6), 5.33 (1H, d, -CH=CH 2 ), 5.63 (1H, d, -CH=CH 2 ), 5.79-5.85 (1H, m, C-7), 6.68 (1H, s, aminothiazole ring-H), 6.89-6.97 (1H, m, -CH=CH 2 ), 7.15 (2H, brds, -NH ...
Embodiment 3
[0024] Get cefdinir crude product 100g, add 10% potassium bicarbonate solution, fully stir until clarification, obtain cefdinir potassium solution, add 6g gac to absorb at room temperature for 20 minutes, filter for decarburization, add 2% acetic acid solution to the filtrate, stir to react, Crystals were precipitated, filtered, washed with water, and dried under reduced pressure at 45°C to obtain 93.1 g of cefdinir refined product with a yield of 93.1% and a purity of 99.8% by HPLC.
[0025] Structural confirmation: Elemental analysis theoretical value C: 42.5%, H: 3.3%, N: 17.7%, O: 20.2%, S: 16.2%; experimental value C: 42.4%, H: 3.5%, N: 17.6%, O : 20.1%, S: 16.3%.
[0026] H-NMR (δ, DMSO-d 6 ): 3.55, 3.86 (2H, Abq, C-2), 5.23 (1H, d, C-6), 5.31 (1H, d, -CH=CH 2 ), 5.64 (1H, d, -CH=CH 2 ), 5.78-5.84 (1H, m, C-7), 6.69 (1H, s, aminothiazole ring-H), 6.89-6.98 (1H, m, -CH=CH 2 ), 7.16 (2H, brds, -NH 2 ), 9.78 (1H, d, -NH-).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com