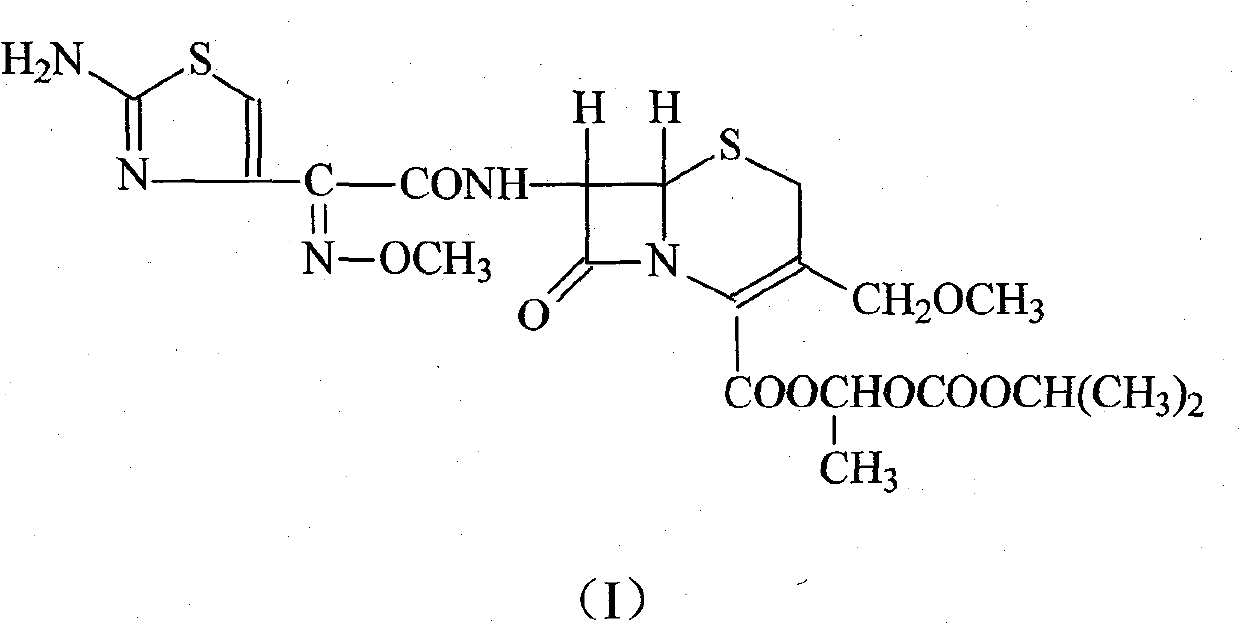
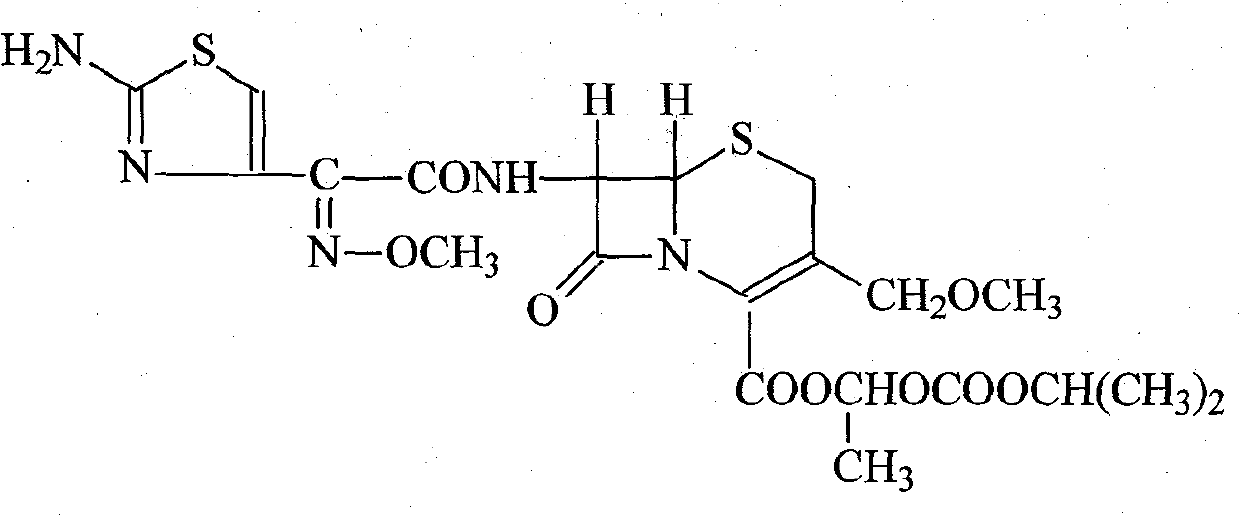
Refining method of Cefpodoxime proxetil compound
A technology of cefpodoxime axetil and refining method, which is applied in the field of medicine to achieve the effects of ensuring safety, improving quality and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] (1) Dissolve 100 g of cefpodoxime axetil crude product in 500 ml of N, N-dimethylformamide, add 180 ml of 2mol / L sodium hydroxide solution, stir and react at room temperature for 60 minutes, hydrolyze to separate crystals, filter, and ethanol Washing and drying under reduced pressure at 50°C gave 96.7 g of cefpodoxime sodium salt, with a yield of 96.7%;
[0021] 1 H-NMR (δ, D 2 O): 3.15(s, 3H, C-OCH 3 ), 3.37 (ABq, 2H, C-2), 3.85 (s, 3H, =N-OCH 3 ), 4.07 (d, 2H, -CH 2 -OCH 3 ), 5.09 (d, 1H, C-6), 5.66 (d, 1H, C-7), 6.88 (s, 1H, aminothiazole ring-H).
[0022] (2) Dissolve the above-mentioned cefpodoxime sodium salt in 800ml of water, add 5g of activated carbon for adsorption for 30 minutes, filter and decarburize, then add 77.4g of 1-iodoethyl isopropyl carbonate and 200ml of tetrahydrofuran, and react with stirring at room temperature for 60 minutes, Then add 800ml of ethyl acetate and 200ml of water, stir and extract, separate layers, wash the organic phase with...
Embodiment 2
[0026] (1) Dissolve 100 g of cefpodoxime axetil crude product in 300 ml of methanol and 500 ml of chloroform, add 50 ml of 5 mol / L sodium acetate solution, stir and react at room temperature for 30 minutes, hydrolyze to precipitate crystals, filter, wash with ethanol, 50 ° C Dry under reduced pressure to obtain 97.2 g of cefpodoxime sodium salt, with a yield of 97.2%;
[0027] 1 H-NMR (δ, D 2 O): 3.16 (s, 3H, C-OCH 3 ), 3.36 (ABq, 2H, C-2), 3.87 (s, 3H, =N-OCH 3 ), 4.06 (d, 2H, -CH 2 -OCH 3 ), 5.10 (d, 1H, C-6), 5.68 (d, 1H, C-7), 6.89 (s, 1H, aminothiazole ring-H).
[0028] (2) Dissolve the above-mentioned cefpodoxime sodium salt in 1000ml of water, add 8g of activated carbon for adsorption for 30 minutes, filter for decarburization, then add 116.1g of 1-iodoethyl isopropyl carbonate and N,N-dimethylacetamide 300ml, stirred at room temperature for 30 minutes, then added 1200ml ethyl acetate and 200ml water, stirred and extracted, layered, the organic phase was washed wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com