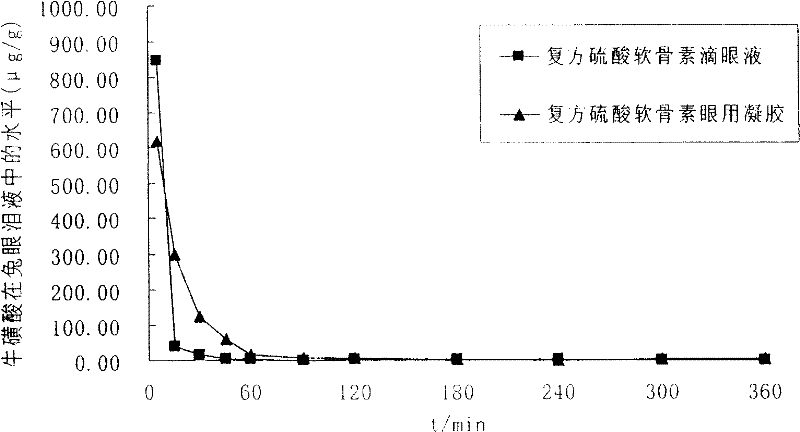
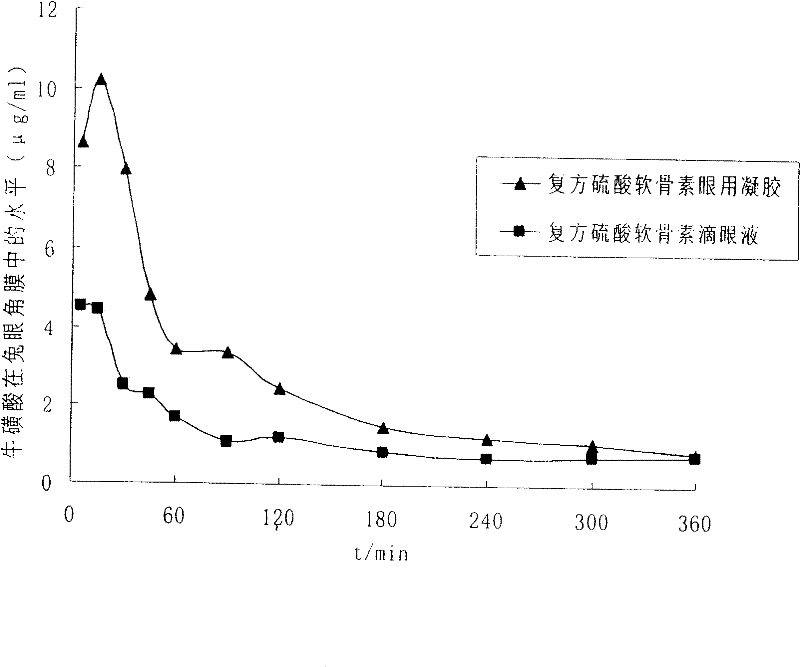
Ophthalmic gel containing chondroitin sulfate and method for preparing same
A technology of chondroitin sulfate and ophthalmic gel, which is applied in the direction of medical preparations containing active ingredients, medical preparations with non-active ingredients, and pharmaceutical formulas, which can solve the problems of short retention time of eye drops, low bioavailability, Weak external stimuli and other issues to achieve the effect of avoiding drug loss, simple preparation process, and stable drug release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1. Preparation of ophthalmic gel of the present invention
[0029] Composition of ophthalmic gel:
[0030] components
content
2.0 grams
taurine
0.25 grams
carbomer
4.0 grams
6.0 grams
5.5 grams
hydrogenated castor oil
10.0 grams
0.10 grams
Co-made
500 g
[0031] Preparation Process:
[0032] Take the prescribed amount of carbomer, add an appropriate amount of water for injection, swell, and sterilize for later use; take an appropriate amount of water for injection, add the prescribed amount of ethylparaben to dissolve, add borax; take another prescribed amount of hydrogenated castor oil, add propylene glycol, Miscible with each other, mix well with the previous step, filter through 0.22 microns and add to carbomer; take the prescribed amount of chondroitin sulfate and taurine, add to dissolve, s...
Embodiment 2
[0033] Embodiment 2. Preparation of ophthalmic gel of the present invention
[0034] Composition of ophthalmic gel:
[0035] components
content
[0036] Chondroitin Sulfate
2.0 grams
taurine
0.25 grams
carbomer
4.5 grams
4.0 grams
glycerin
4.2 grams
hydrogenated castor oil
8.0 grams
0.12 grams
Co-made
500 g
[0037] Preparation Process:
[0038] Take the prescribed amount of carbomer, add an appropriate amount of water for injection, swell, and sterilize for later use; take an appropriate amount of water for injection, add the prescribed amount of benzalkonium chloride to dissolve, and add sodium hydroxide; Glycerin, miscible with each other, mixed with the previous step, filtered through 0.22 microns and added to carbomer; take the prescribed amount of chondroitin sulfate and taurine, add and dissolve, stir well, add t...
Embodiment 3
[0039] Embodiment 3. Preparation of ophthalmic gel of the present invention
[0040] Composition of ophthalmic gel:
[0041] components
content
2.0 grams
taurine
0.25 grams
carbomer
3.5 grams
1.5 grams
3.5 grams
2.0 grams
hydrogenated castor oil
10.0 grams
0.10 grams
Co-made
500 g
[0042] Preparation Process:
[0043] Take the prescribed amount of carbomer and hypromellose, add an appropriate amount of water for injection, swell, and sterilize for later use; take an appropriate amount of water for injection, add the prescribed amount of benzalkonium bromide to dissolve, add sodium dihydrogen phosphate; take another prescription A certain amount of hydrogenated castor oil, add mannitol, miscible with each other, mix with the previous step, filter through 0.22 microns ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com