Liposome calcium sulphate composite nano artificial bone, preparation method and use thereof
A liposome and artificial bone technology, applied in the field of medical materials, can solve the problems of rapid degradation and absorption, poor osteoinductive ability, high price, etc., and achieve the effect of long usable time, meeting requirements, and high material strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Weigh 15g of lecithin and 12g of cholesterol, dissolve in 50ml of dichloromethane, oscillate evenly to obtain solution I, then put it into an eggplant-shaped bottle, distill under reduced pressure on a rotary evaporator, and feed nitrogen from time to time until the dichloromethane The methane is completely evaporated, and then vacuum-dried at room temperature for 24 hours; weigh 3.0g bone morphogenic protein (BMP) and dissolve it in 70ml PBS (pH=7.4) buffer solution and shake well to obtain the aqueous solution II; then add the above 70ml BMP solution II into the eggplant-shaped bottle , under the conditions of ultrasound to fully hydrate the lipid film, after ultrasound, to obtain liposomes loaded with BMP, freeze-dried as a powder for future use.
[0045] Incubate calcium sulfate dihydrate at 100°C for 5 hours to generate calcium sulfate hemihydrate; mix 80g calcium sulfate hemihydrate, 10g calcium sulfate dihydrate and 10g α-tricalcium phosphate, and grind in a ball ...
Embodiment 2
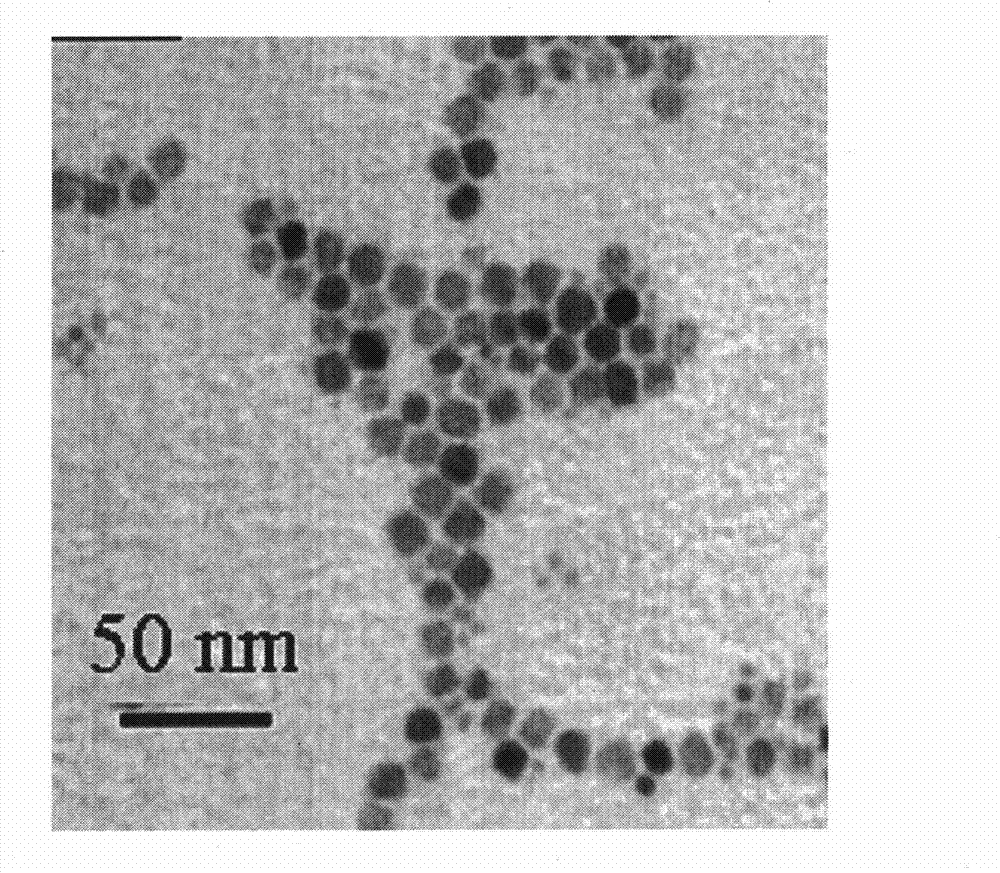
[0048] Take by weighing carboxymethyl chitosan octadecyl quaternary ammonium salt (weight average molecular weight is 2,000,000) 15g, cholesterol 12g is dissolved in 50ml dichloromethane, shakes evenly to obtain solution I; Take by weighing 3.0g gentamicin to dissolve The aqueous solution II was obtained in 70ml of deionized water; then the above two solutions I and II were blended and emulsified, ultrasonicated for 10 minutes, and put into an eggplant-shaped bottle after forming a stable emulsion, and the dichloromethane was evaporated on a rotary evaporator. The polymer liposome aqueous solution loaded with gentamicin, figure 1 It is the TEM photo of the polymer liposome loaded with gentamicin.
[0049] Keep calcium sulfate dihydrate at 1200°C for 4 hours, take it out, and quench it in an ice bath to form anhydrous calcium sulfate. Grind 100 g of anhydrous calcium sulfate in a ball mill for 12 hours to obtain calcium sulfate powder.
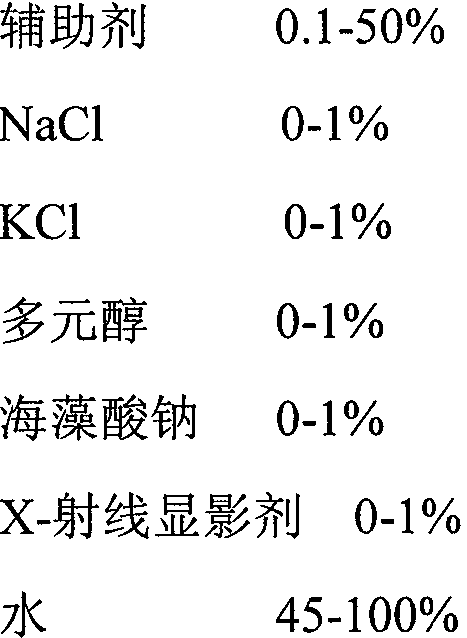
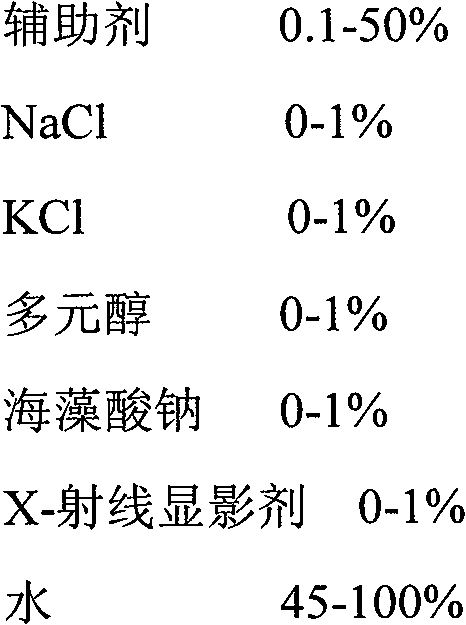
[0050] The diluent is an aqueous soluti...
Embodiment 3
[0052] Take by weighing carboxymethyl chitosan octadecyl quaternary ammonium salt (weight average molecular weight is 1000) 15g, cholesterol 12g, 2.0g carbon nanotubes are dissolved in 50ml dichloromethane, shake to obtain solution I evenly; Weigh 3.0g Dissolve BMP in 70ml deionized water to obtain aqueous solution II; then blend and emulsify the above two solutions I and II, ultrasonicate for 10 minutes, put it into an eggplant-shaped bottle after forming a stable emulsion, and volatilize dichloromethane on a rotary evaporator After cleaning, liposomes co-carrying carbon nanotubes and BMP are obtained, and freeze-dried as a powder for future use.
[0053] Calcium sulfate dihydrate is incubated at 120°C for 8 hours to generate calcium sulfate hemihydrate, and calcium sulfate dihydrate is incubated at 1200°C for 2 hours to generate calcium sulfate anhydrous; 30g calcium sulfate dihydrate, 10g calcium sulfate hemihydrate, and 60g anhydrous sulfuric acid Calcium and 5g of tetraca...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| strength | aaaaa | aaaaa |
| compressive strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com