Method for synthesizing photochromic cross-linked polymer containing naphthyl hydroxide pyran group
A cross-linked polymer and a technology containing naphthol pyran, which is applied in the field of cross-linked polymer synthesis, can solve the problems of no naphthol pyran photochromic cross-linked polymer material report, no material characterization, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
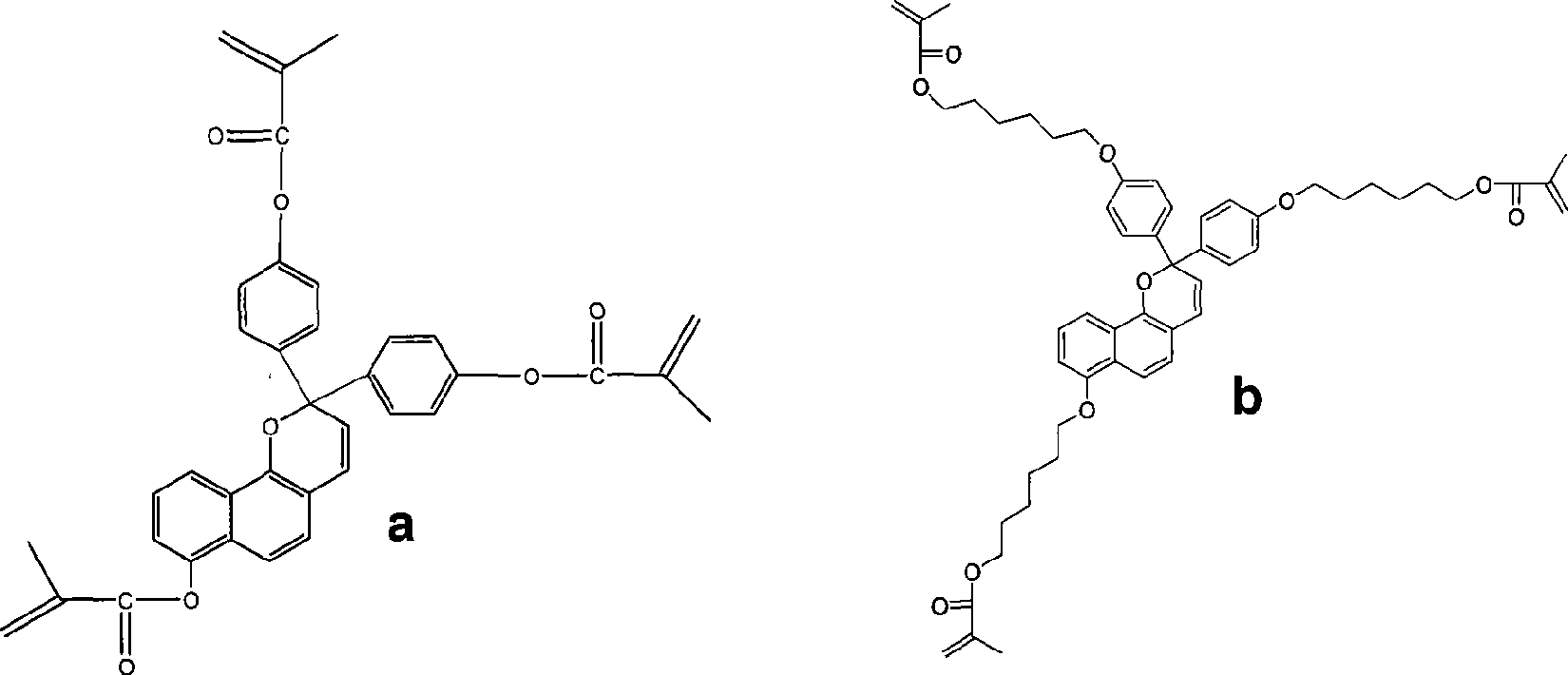
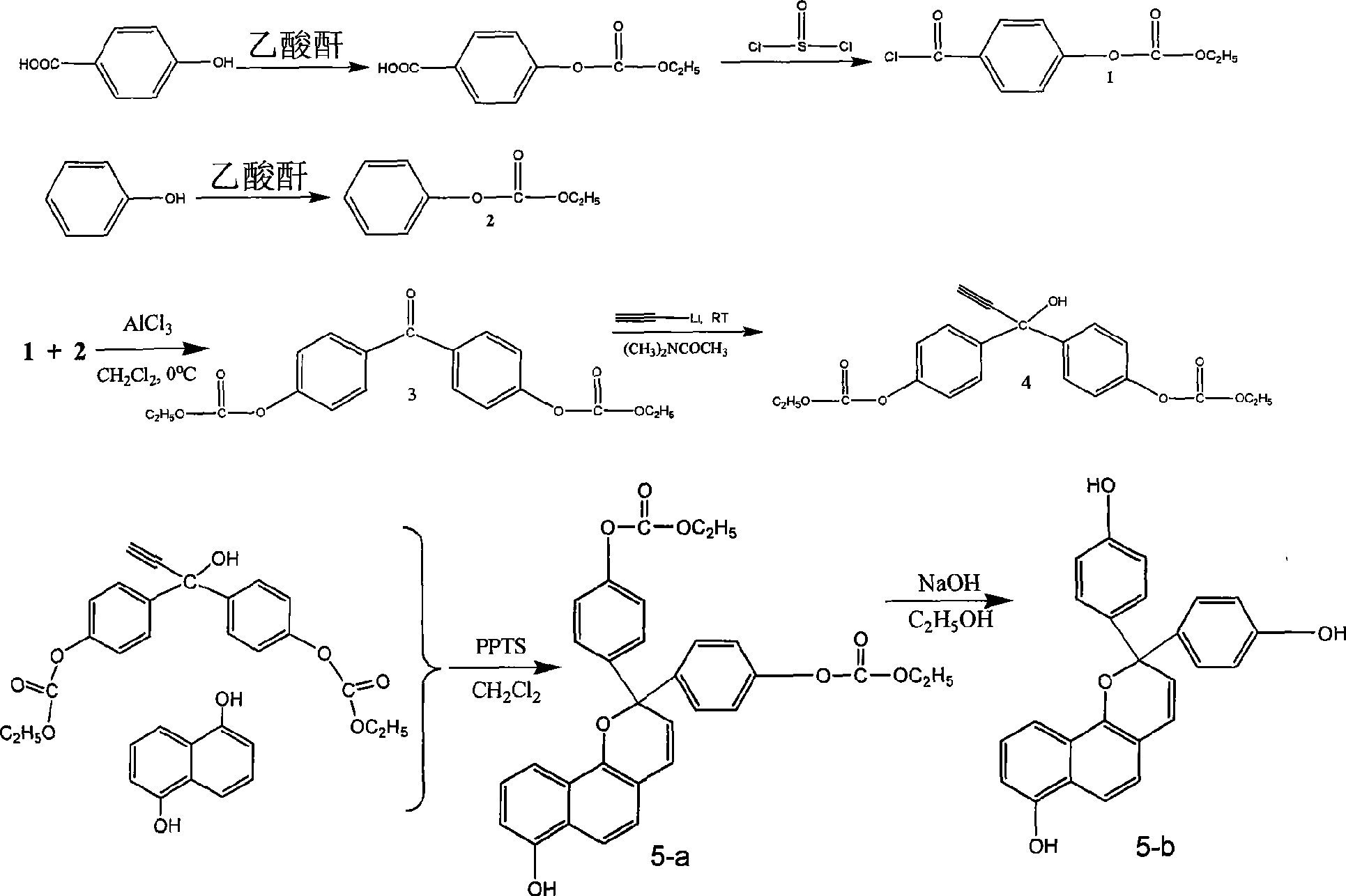
[0028] Embodiment 1: the preparation of monomer a, reaction formula is as follows:
[0029]
[0030]
[0031] In the reaction bottle, add 10g of p-hydroxybenzoic acid and 50mL of acetic anhydride, heat and reflux for 6 hours, distill off the acetic anhydride, add 100mL of water to the residue, then extract three times with chloroform, combine the chloroform solution, wash the chloroform solution with water, anhydrous sodium sulfate dry. Column chromatography separated a white solid, 8.8g p-carboxyphenol acetate.
[0032] Dissolve 8.5 g of p-carboxyphenol acetate in 50 mL of thionyl chloride, heat to reflux for 5 hours, then distill off excess thionyl chloride, and distill off 7.3 g of acid chloride compound 1 prepared by the reaction under reduced pressure.
[0033] Preparation of compound 2:
[0034] 10g of phenol was added to 50mL of acetic anhydride, heated to reflux for 8 hours, and then excess acetic anhydride was distilled off. 100 mL of water was added to the r...
Embodiment 2
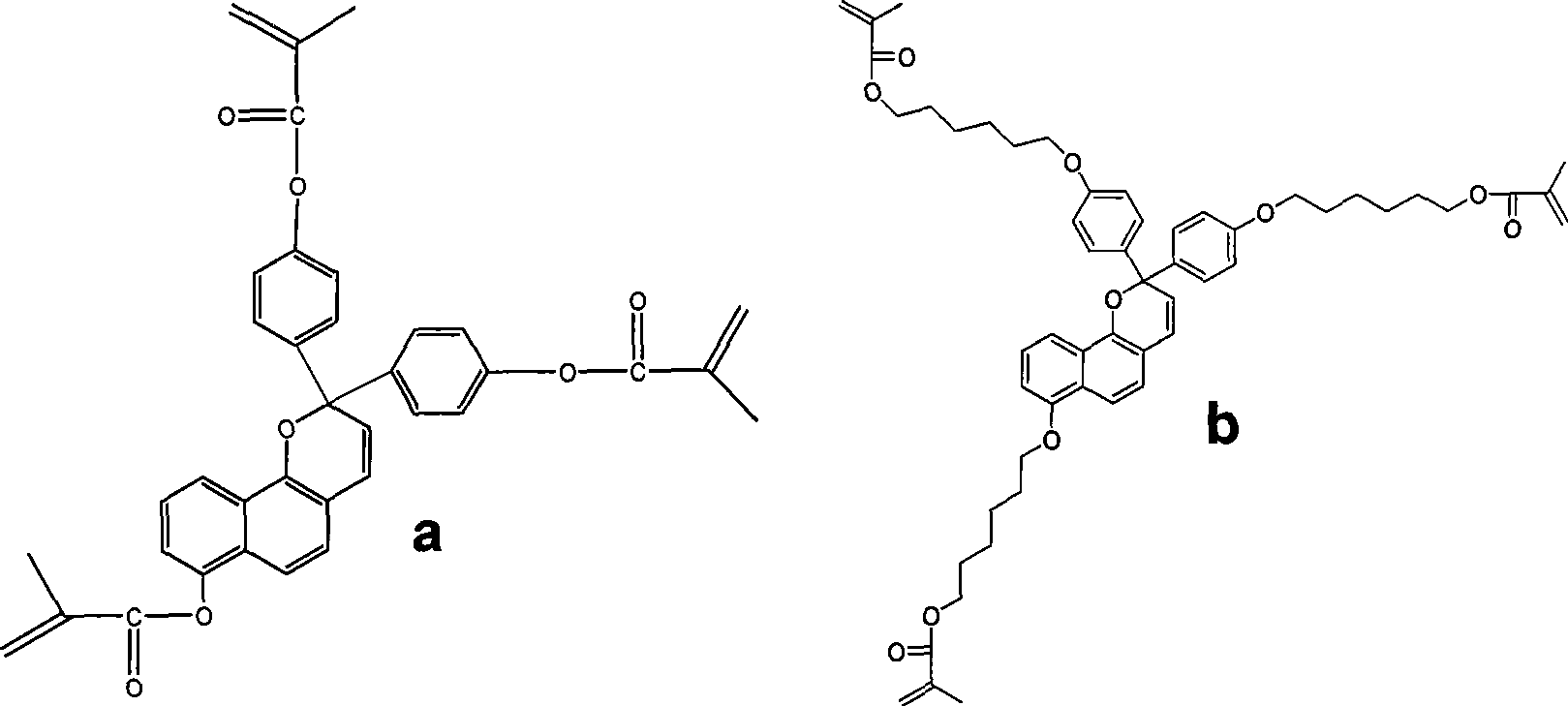
[0044] Embodiment 2: the preparation of monomer b, the reaction formula is as follows:
[0045]
[0046]
[0047] Specific implementation reaction example:
[0048] Preparation of Compounds 6 and 7:
[0049] In the reaction flask, 10.0 g of phenol, 20.0 g of potassium carbonate and a small amount of KI were added to 50 mL of DMF, stirred, and heated to 85° C. for 0.5 hours to react. Then add 20.0g 1-bromohexanol, continue to react for 12 hours, add 250mL water after cooling down to room temperature. Then it was extracted three times with dichloromethane, the dichloromethane solution was combined, the dichloromethane solution was washed with water, and dried over anhydrous sodium sulfate. Compound 6 was separated by column chromatography, 17.5 g, with a yield of 85%.
[0050] In a 150 mL round bottom flask, 16.0 g of compound 6 was added to 50 mL of acetic anhydride, heated to reflux for 8 hours, and then excess acetic anhydride was distilled off. 100 mL of water was ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| dispersity | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com