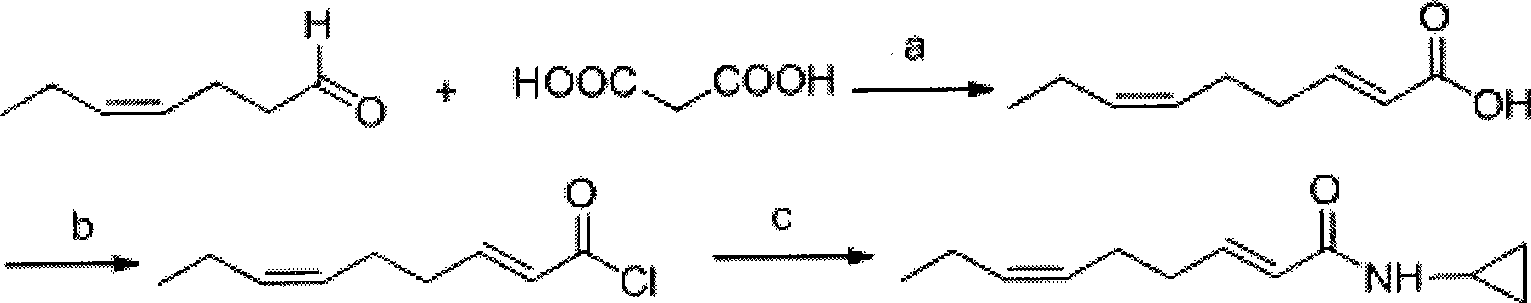
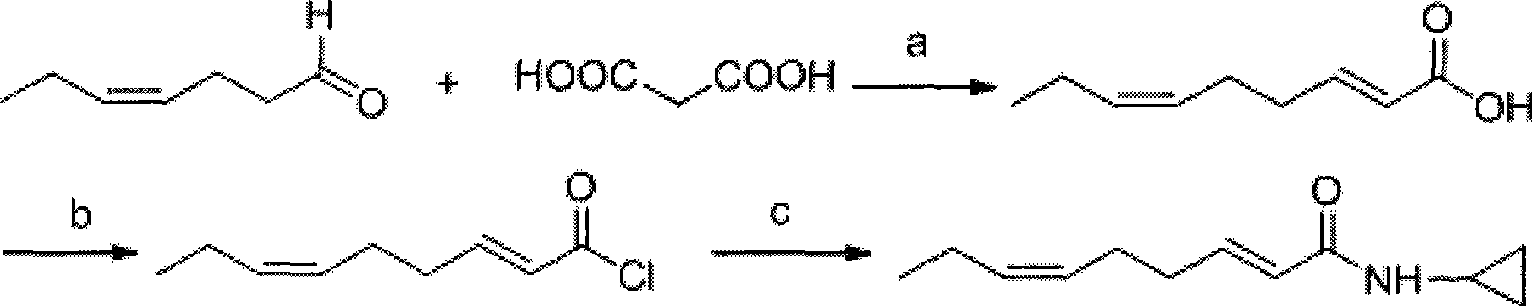
Method for synthesizing N-cyclopropyl-trans-2-cis-6-nonadienoic acid acidamide
A technology for the synthesis of nonadienyl acid amides, which is applied to the preparation of carboxylic acid amides, chemical instruments and methods, and pharmaceutical formulations. It can solve problems such as complex operations and low reaction yields, and achieve high product yields and excellent reaction conditions mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Step 1: Preparation of trans-2-cis-6-nonadienoic acid
[0029] In a 500mL three-necked flask equipped with a stirrer, a thermometer and a condenser tube, add 22.4g (0.2mol) cis-4-heptenal, 41.6g (0.4mol) malonic acid and 100mL pyridine, and add Inject 2.5mL of hexahydropyridine, raise the temperature to 65°C, react for 4h, TLC detects that the reaction is complete, cool the reaction solution, pour it into a mixture of ice and water containing 500mL of 1N concentrated hydrochloric acid, extract with ether, and wash twice with water. The organic phase was distilled under normal pressure to recover diethyl ether, and the fraction at 102-103° C. / 0.5 mmHg was collected through vacuum distillation to obtain 26.2 g of the product with a yield of 85%.
[0030] The second step: the preparation of trans-2-cis-6-nonadienoyl chloride intermediate product:
[0031] In a 500mL three-necked flask equipped with a stirrer, a thermometer, a dropping funnel and an exhaust gas absorption ...
Embodiment 2
[0035] Step 1: Preparation of trans-2-cis-6-nonadienoic acid
[0036] In a 500mL three-necked flask equipped with a stirrer, a thermometer and a condenser tube, add 22.4g (0.2mol) cis-4-heptenal, 20.8g (0.2mol) malonic acid, 20.4g (0.2mol) basic oxidation Aluminum and 100mL chloroform were heated to reflux for 7 hours. TLC detected that the reaction was complete. Cool the reaction solution, filter off alumina, pour it into a mixture of ice and water containing 500mL of 1N concentrated hydrochloric acid, extract with ether, and wash twice with water. The organic phase was distilled under normal pressure to recover diethyl ether, and the fraction at 102-103° C. / 0.5 mmHg was collected through vacuum distillation to obtain 22.0 g of the product with a yield of 71%.
[0037] The second step: the preparation of trans-2-cis-6-nonadienoyl chloride:
[0038] In a 500mL three-necked flask equipped with a stirrer, a thermometer, a dropping funnel and an exhaust gas absorption device, ad...
Embodiment 3
[0041] Step 1: Preparation of trans-2-cis-6-nonadienoic acid
[0042] In a 500mL three-necked flask equipped with a stirrer, a thermometer and a condenser tube, add 22.4g (0.2mol) cis-4-heptenal, 20.8g (0.2mol) malonic acid, 2.5mL aniline and 100mL chloroform, and heat to reflux After reacting for 7 hours, TLC detected that the reaction was complete, cooled the reaction solution, poured it into a mixture of ice and water containing 500 mL of 1N concentrated hydrochloric acid, extracted with ether, and washed twice with water. The organic phase was distilled under normal pressure to recover diethyl ether, and the fraction at 102-103° C. / 0.5 mmHg was collected through vacuum distillation to obtain 24.6 g of the product with a yield of 80%.
[0043] The second step: the preparation of trans-2-cis-6-nonadienoyl chloride:
[0044] In a 500mL three-necked flask equipped with a stirrer, a thermometer, a dropping funnel and an exhaust gas absorption device, add 15.4 g (0.1 mol) of tr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com