Application of 4-acetyl diazene quinoline derivatives in preparing anti-tumor medicament
A technology for acetyldiazepine quinoline and antitumor drug, which is applied in the application field of 4-acetyldiazepine quinoline derivatives in the preparation of anti-cancer drugs, can solve the problems of adverse reactions of antitumor drugs and the like, and achieves difficult tolerance Medicinal properties, obvious effect, obvious effect on tumor inhibition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
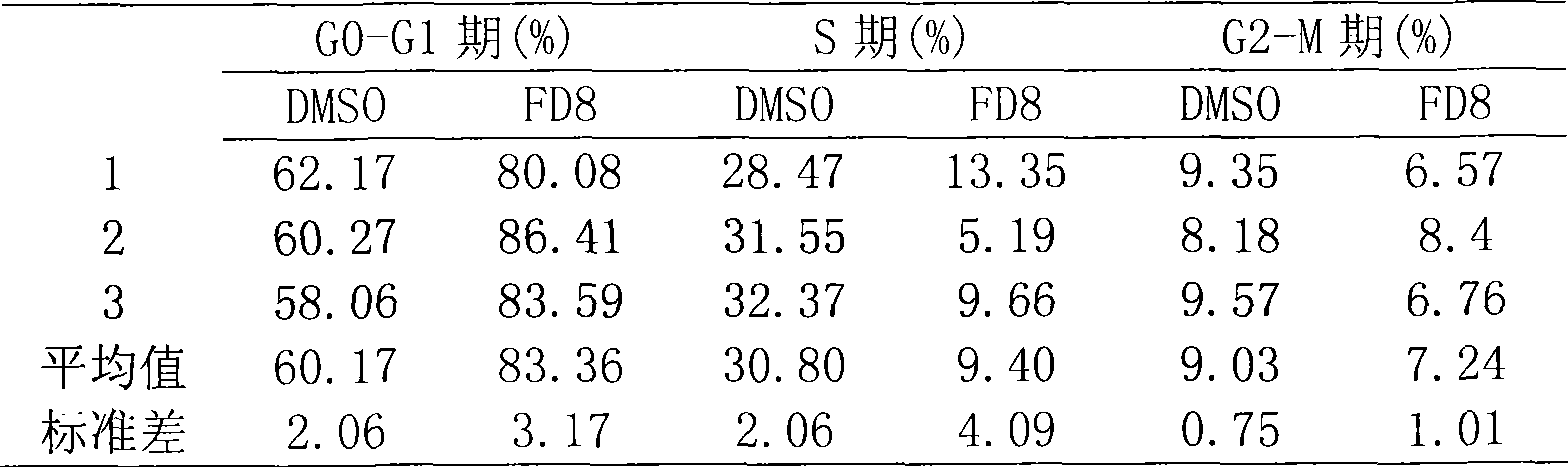
Image
Examples
Embodiment 1
[0025] Example 1 Virtual Screening of CypJ Small Molecule Inhibitors
[0026]In the PDB protein structure database, the X-ray diffraction crystal structure of the human CYPA protein was retrieved (PDB code: 1CWA). This structure is the crystal structure of CYPA in complex with its natural inhibitor cyclosporin A (CsA). From this structure, the active site of CYPA was determined, and several key amino acid sites in the active site that could be inhibited by CsA were determined. According to the highly homologous sequence between CYPJ and CYPA, we cooperated with the Shanghai Institute of Materia Medica, Chinese Academy of Sciences to establish a 3D structure model, which was also confirmed in subsequent protein crystallization and structural analysis (CYPJ PDB code: 1XYH). Several small molecule databases were screened against the CypJ active site. The small molecule databases used for screening mainly include SPECS and CNPD. Finally, FD8 of the present invention was screene...
Embodiment 2
[0027] Example 2 Utilizes BIAcore Molecular Interaction Instrument to Verify Virtual Screening Results
[0028] The BIAcore molecular interaction instrument is based on surface plasmon resonance technology to track the interaction between biomolecules without any markers, thus ensuring the authenticity of the experimental results to the greatest extent. During the experiment, the target biomolecules (CypJ protein) were immobilized on the surface of the sensor chip, and then the small molecule compounds were dissolved in a solvent and flowed over the surface of the chip. The monitor can track the changes in the whole process of binding and dissociation between molecules in the detection solution and target biomolecules on the chip surface in real time. Through the binding data of BIAcore, we finally calculated the 1-[2-(3-ethoxyphenyl)quinolin-4-yl]2-{[4-methoxyl group-3-(morpholine methyl) of the present invention Base) phenyl] diazene} ethyl ketone and CypJ binding equilibri...
Embodiment 3
[0029] Example 3 Use of Enzyme Activity Experiments to Prove the Ability of Small Molecular Compounds to CypJ Enzyme Activity Inhibition
[0030] There are many methods for measuring CyP activity, but α-chymotrypsin-coupled enzymic assay is the most commonly used. The principle is that the oligopeptide substrate containing proline, such as N-succinyl-Ala-Ala-Pro-Phe p-nitroanilide (N-Succinyl-Ala-Ala-Pro-Phep-nitroanilide) in the solution The formula and trans structures are in balance, and CyP can catalyze the cis-trans isomerization of the substrate, that is, catalyze the change of proline from cis to trans; when it is in the trans structure, the C-terminal p-nitroanilide is α- The chymotrypsin is cleaved to release the pigment group p-nitroaniline, and the PPIase activity of CyP can be obtained by continuously measuring the change of the absorbance value at 390nm.
[0031] We take the reaction without small molecule inhibitor as the control reaction, measure the inhibitory...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com