Compositions and methods for dermally treating pain
A skin and drug technology, applied in the field of preparations for the treatment of musculoskeletal pain or neuropathic pain, can solve the problems of expensive storage capsules, hindering skin stretching, discomfort, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
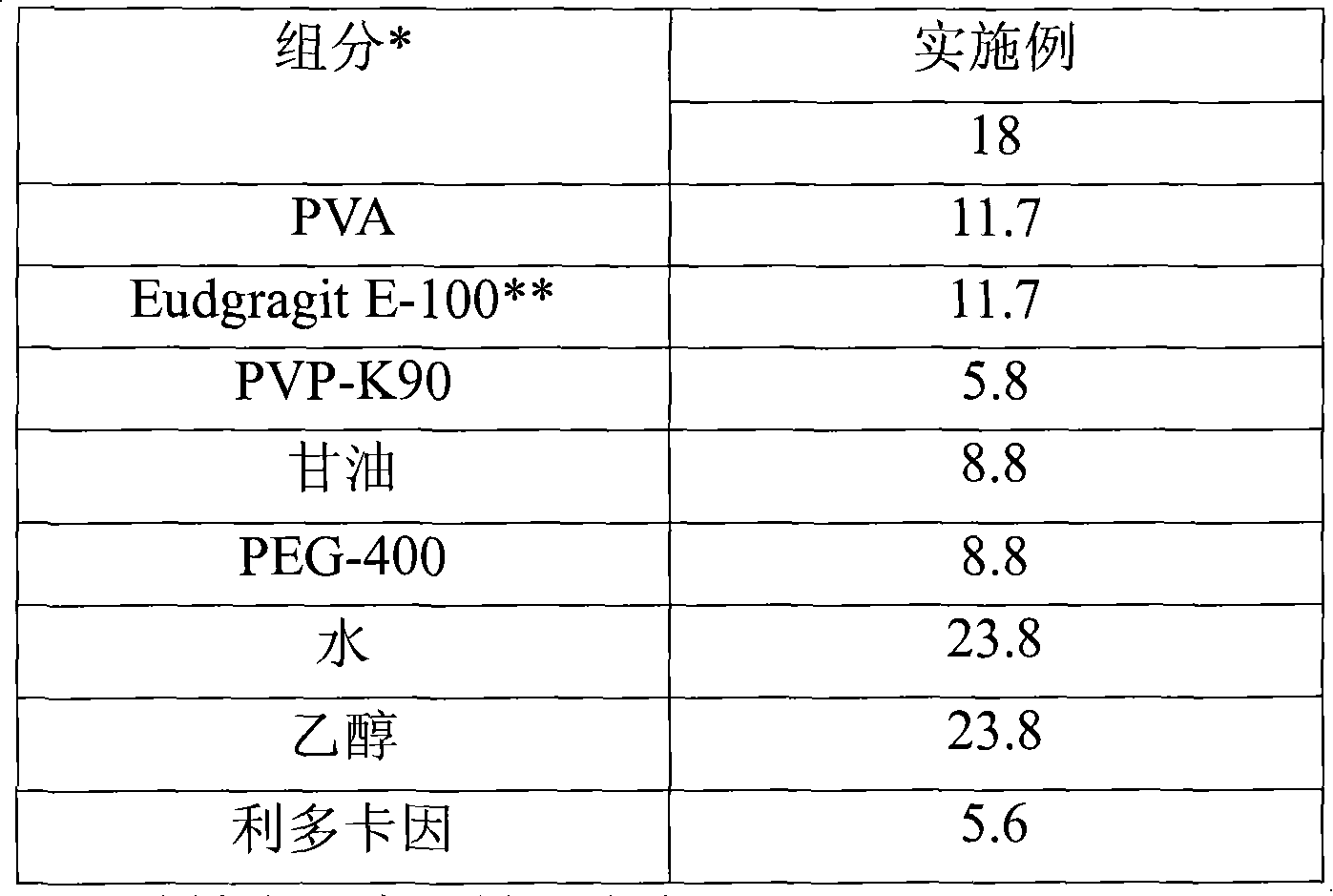
Embodiment 1
[0099] As noted, hairless mouse skin (HMS) or human epidermal membrane (HEM) were used as model membranes for the in vitro flux studies described here. Hairless mouse skin (HMS) was used as a model membrane for the in vitro flux study described here. The newly isolated epidermis from the abdomen of hairless mice was carefully placed between the supply chamber and the receiving chamber of the Franz diffusion cell. The receiving chamber was filled with phosphate buffered saline (PBS) of pH 7.4. The experiment was initiated by placing the test formulation on the stratum corneum (SC) of the skin sample. The Franz cell was placed on a heating table maintained at 37°C and the temperature of the HMS was maintained at 35°C. At preset time intervals, aspirate 800 μL aliquots and replace them with fresh PBS solution. Determine the skin flux (μg / cm) from the steady-state slope of the cumulative penetration versus time curve 2 / h). The placement of the skin and the sampling technique u...
Embodiment 2
[0101] Non-volatile solvent system
[0102] By placing 200 mcL on the stratum corneum side (supply cell) of hairless mouse skin, the steady-state flux of ropivacaine base in the above-mentioned non-volatile solvent was obtained. The in vitro study was performed as described in Example 1. According to Table 2, the non-volatile solvents glycerin and Tween 20 have low steady-state flux values and are not considered "flux favorable". However, mineral oil and isostearic acid are flux-favorable solvents, and are good candidates for evaluating curing agents and volatile solvents to design acceptable release formulations. Surprisingly, Span 20 has a much higher steady-state flux value and will also be qualified as a highly flux-favorable solvent.
Embodiment 3
[0104] Non-volatile solvent system
[0105] By placing 200 mcL on the stratum corneum side (supply cell) of hairless mouse skin, a steady-state flux of diclofenac sodium from the above-mentioned non-volatile solvent was obtained. The in vitro study was performed as described in Example 1. According to Table 3, the non-volatile solvent glycerin has a value of 1mcg / cm 2 The estimated treatment steady-state flux value of / h is equivalent to the steady-state flux value and can be considered as a solvent with favorable flux. However, the steady-state flux values of isopropyl myristate, ethyl oleate, propylene glycol, and Span 20 are at least 10 times the reported flux value of glycerol and are considered favorable for flux.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com