Flavone lignose compound, and preparation and pharmaceutical use thereof
A technology of flavonoid lignin and compound, applied in the field of medicine, can solve the problems of insufficient water solubility and bioavailability, restricting the drug market, etc., and achieve the effects of being conducive to large-scale production, easy availability of raw material sources, and huge social benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
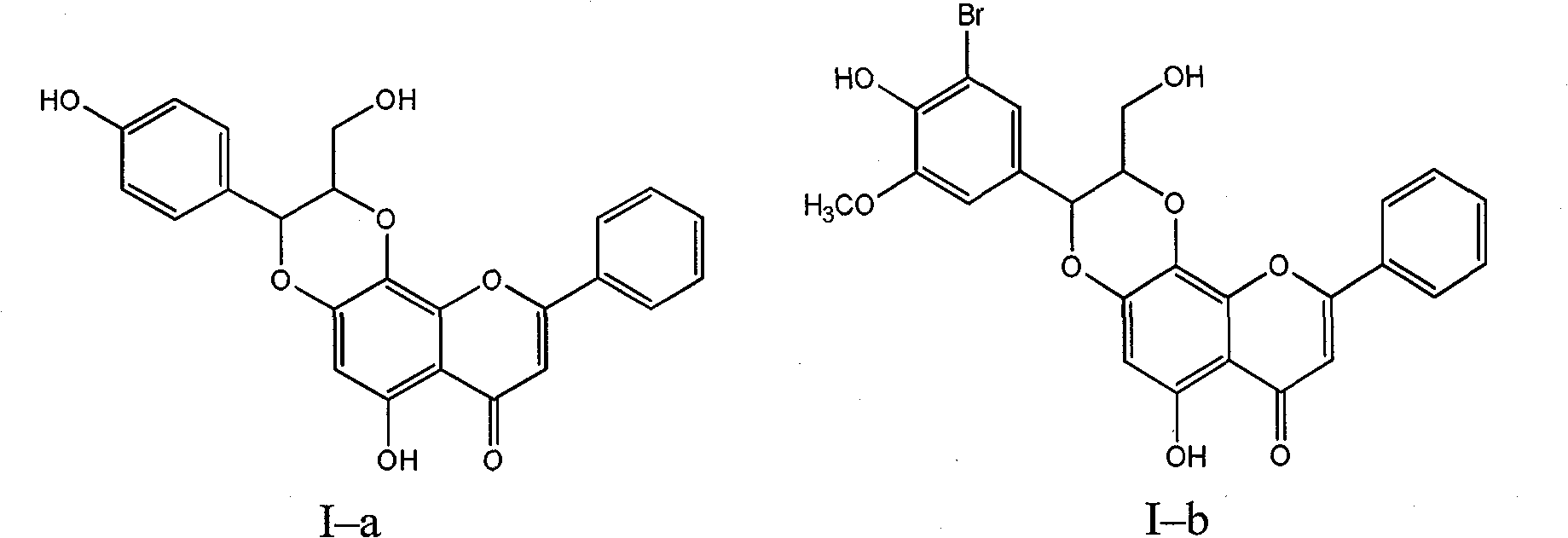
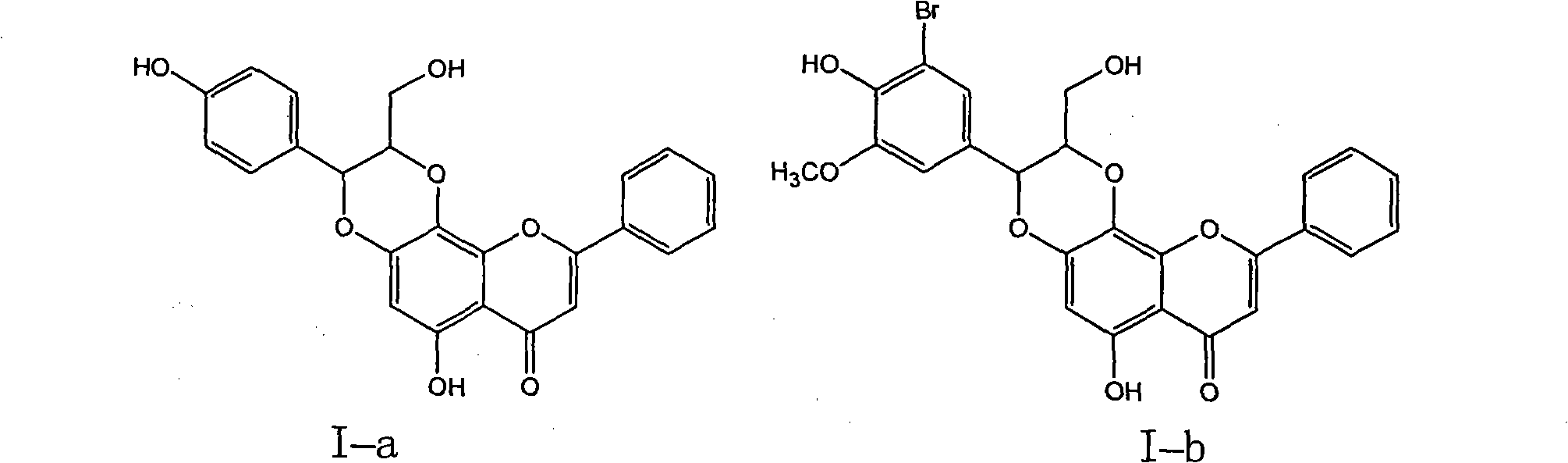
[0030] Example 1 : Compound I-a is (±)-trans-6-hydroxyl-2-hydroxymethyl-3-(4-hydroxyphenyl)-9-phenyl-2,3-dihydro-[1,4]dioxane Preparation of cyclo[2,3-h]benzopyran-7-one
[0031]
[0032] 1.1 Put 2.28 grams of 5,7-dihydroxyflavone into a dry nitrogen-protected reaction flask, dissolve it in 3.15 grams of potassium hydroxide aqueous solution, add 0.322 grams of tetrabutylammonium bromide, and add the dissolved 3 g of potassium persulfate in 90 ml of aqueous solution, after the dropwise addition, was stirred for 2 hours, the ice bath was removed, and left overnight at room temperature. Use 1N hydrochloric acid to adjust the pH value to 5, stir, filter the precipitated khaki solid, add 22.5 ml of concentrated hydrochloric acid and 2.25 g of sodium sulfite to the filtrate, heat and boil for 10 minutes, let stand to cool, filter, wash the filter cake with water until neutral, and dry to obtain 1.07 g of brown-yellow crude product was recrystallized from absolute ethanol to obt...
Embodiment 2
[0035] Example 2 : Compound I-b is (±)-trans-6-hydroxyl-2-hydroxymethyl-3-(3-bromo-4-hydroxyl-5-methoxyphenyl)-9-phenyl-2,3-di Preparation of Hydrogen-[1,4]dioxane[2,3-h]benzopyran 7-one
[0036]
[0037] 2.15, The preparation of 7,8-trihydroxyflavone, the method is the same as described in 1.1 in Example 1.
[0038] 2.2 Put 0.22 g of 5,7,8-trihydroxyflavone, 0.423 g of 3-bromo-4-hydroxy-5-methoxyl-cinnamyl alcohol and 0.56 g of silver oxide into a reaction flask protected by dry nitrogen, and inject 8 ml of anhydrous Acetone and 24 ml of anhydrous benzene were incubated at 50-60°C for 15 hours, filtered, concentrated mother liquor, and separated by column chromatography on silica gel (200-300 mesh, 20 g) (chloroform / methanol=9:1-2:1) , to obtain 0.193 g of light yellow needle-like crystals, with a yield of 45%.
[0039] (±)-trans-6-hydroxyl-2-hydroxymethyl-3-(3-bromo-4-hydroxyl-5-methoxyphenyl)-9-phenyl-2,3-dihydro-[1 , 4] dioxane [2,3-h] benzopyran-7-one (I-b): R f ...
Embodiment 3
[0045] Example 3 : Compound I-b is (±)-trans-6-hydroxyl-2-hydroxymethyl-3-(3-bromo-4-hydroxyl-5-methoxyphenyl)-9-phenyl-2,3-di Hydrogen-[1,4]dioxane[2,3-h]benzopyran-7-one to hydrogen peroxide H 2 o 2 Protective effect of induced PC12 cell injury
[0046] 3.1 Experimental materials and samples
[0047] 3.1.1 Cells: Rat adrenal pheochromoma cells (PC12) were purchased from Shanghai Institute of Cells, Chinese Academy of Sciences.
[0048] 3.1.2 Experimental reagents:
[0049] 3.1.2.1 Hydrogen peroxide (H 2 o 2 ), nitroblue tetrazolium (NBT), and phenanthrozine (ferrozine) were purchased from Sigma;
[0050] 3.1.2.2 Quercetin (quercetin) was provided by the Laboratory of Traditional Chinese Medicine and Natural Medicine, School of Pharmacy, Zhejiang University (purity: 99%); silybin (Silybin) was purchased from Liaoning Panjin Tianyuan Pharmaceutical Co., Ltd., and detected by HPLC 98% purity.
[0051] 3.1.2.3 Tris base, DMEM medium was purchased from Gibco;
[0052] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| control rate | aaaaa | aaaaa |
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com