Application of isosulfocyanate compound in preparing medicament for treating leukemia
A technology of ester compound, benzyl isothiocyanate, which is applied in the field of isothiocyanate compounds, can solve the problems of high cost of treatment, lack of access to patients, and inability of bone marrow to pay for treatment.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
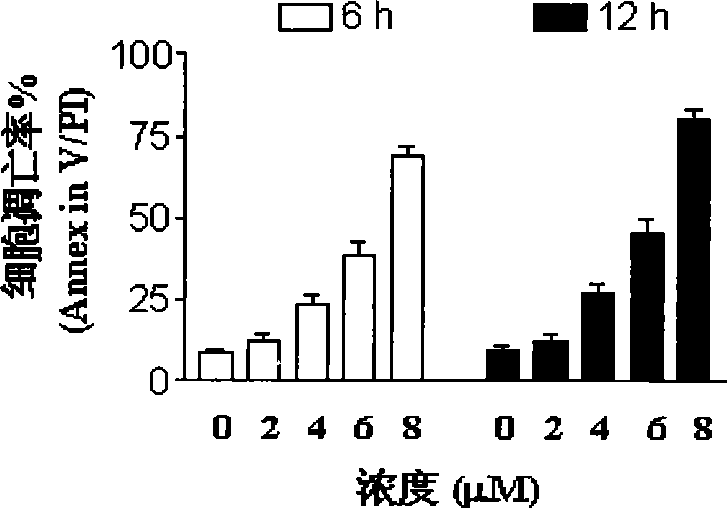
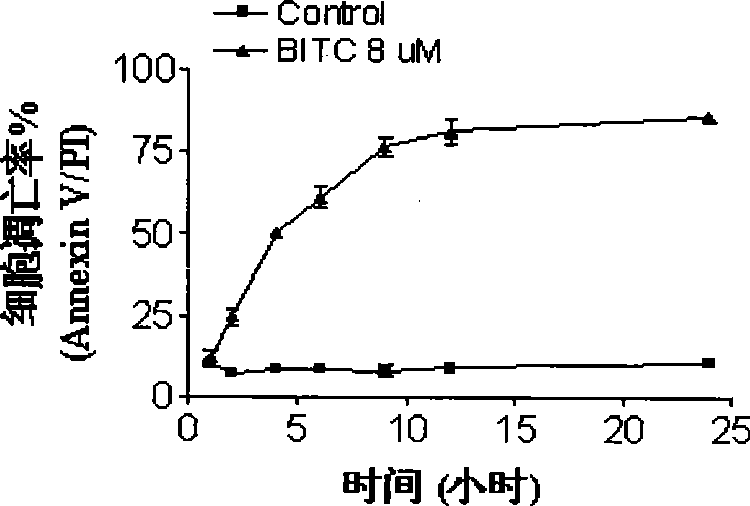
[0054] Peripheral blood cells or bone marrow were collected from 6 cases of acute myeloid leukemia (AML), 3 cases of acute lymphoblastic leukemia (ALL) and 3 cases of chronic myeloid leukemia (CML), and mononuclear cells were isolated. At the same time, the bone marrow of 4 normal people was collected and hematopoietic stem cells (CD34 + ). AnnexinV / PI double staining method and flow cytometry were used to detect the occurrence of apoptosis after the cells were treated with five kinds of isothiocyanate compounds in the present invention. The concentrations of the five compounds were: BITC 8 μM, AITC 20 μM, PEITC 8 μM , SFN 40μM, Erucin 20μM,; the action time is 24 hours. See the results of the study Figure 8-1A , Figure 8-2A , Figure 8-3A , Figure 8-4A , Figure 8-5A , Figure 8-1B , Figure 8-2B , Figure 8-3B , Figure 8-4B , Figure 8-5B , Figure 8-1C , Figure 8-2C , Figure 8-3C , Figure 8-4C ,and Figure 8-5C , the results showed that the five isoth...
Embodiment 2
[0056] Sixty female NOD / SCID mice aged 4-5 weeks were selected to establish a xenograft model of NOD / SCID mice with acute leukemia U937 cells. NOD / SCID mice were subcutaneously inoculated with 0.2ml acute leukemia U937 cell suspension (containing about 2×10 cells) on the right back. 63 days after cell inoculation, the mice were randomly divided into control group, BITC treatment group, and PEITC treatment group according to body weight. Mice in the control group were intraperitoneally injected with normal saline; mice in the treatment group were injected intraperitoneally with BITC and PEITC at a dose of 12 μmol / day, 3 times a week for 4 weeks. The results showed that the incidence of tumors in the BITC and PEITC treatment groups were 42.5% and 45%, respectively, while the incidence of tumors in the normal saline control group was 87.5% (see Figure 8-1E and Figure 8-3E ); the tumor weights of BITC and PEITC treatment groups were 1.33±1.90 and 1.30±1.87, and those of the co...
Embodiment 3
[0058] Prepare isothiocyanate capsules by the well-known capsule method of those skilled in the art; Wherein said capsule can be made to contain 50% benzyl isothiocyanate (BITC), benzene isothiocyanate Capsules of ethyl ethyl ester (PEITC), allyl isothiocyanate (AITC), sulforaphane (SFN) or glycoside trierucinate (Erucin) can also increase or decrease isothiocyanate Benzyl ester (BITC), phenylethyl isothiocyanate (PEITC), allyl isothiocyanate (AITC), sulforaphane (SFN) or glycoside trierucin (Erucin) content . Using commercially available isothiocyanate compounds, get 1500 grams of benzyl isothiocyanate (BITC), phenylethyl isothiocyanate (PEITC), allyl isothiocyanate (AITC), Sulforaphane (SFN) or Trierucin (Erucin), 418g soybean oil, 400g gelatin, 400g purified water, 70g beeswax, 10g red iron oxide, 10g black iron oxide, 2g Ethylparaben, 200 grams of glycerin, prepared into capsules according to the known capsule preparation technology, that is: adopting soybean oil and bee...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com