Rechargeable magnesium cell anode material and preparation thereof
A cathode material and technology for magnesium batteries, which are applied in battery electrodes, chemical instruments and methods, circuits, etc., can solve the problems of unsatisfactory cathodes and poor oxidation stability of rechargeable magnesium batteries, so as to reduce synthesis costs and shorten reactions. Period, the effect of speeding up the reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] 0.6g of KCl, 0.0968g of MgO, 0.4354g of FeC 2 o 4 2H2 O and 0.1452g of SiO 2 After grinding for 30 minutes until uniform, vacuum-dry at 100°C for 12 hours, then heat-treat at 350°C for 2 hours under a protective atmosphere of argon, then heat-treat at 900°C for 6 hours, and finally cool naturally to room temperature to obtain a rechargeable Magnesium iron magnesium silicate is a cathode material for magnesium batteries.
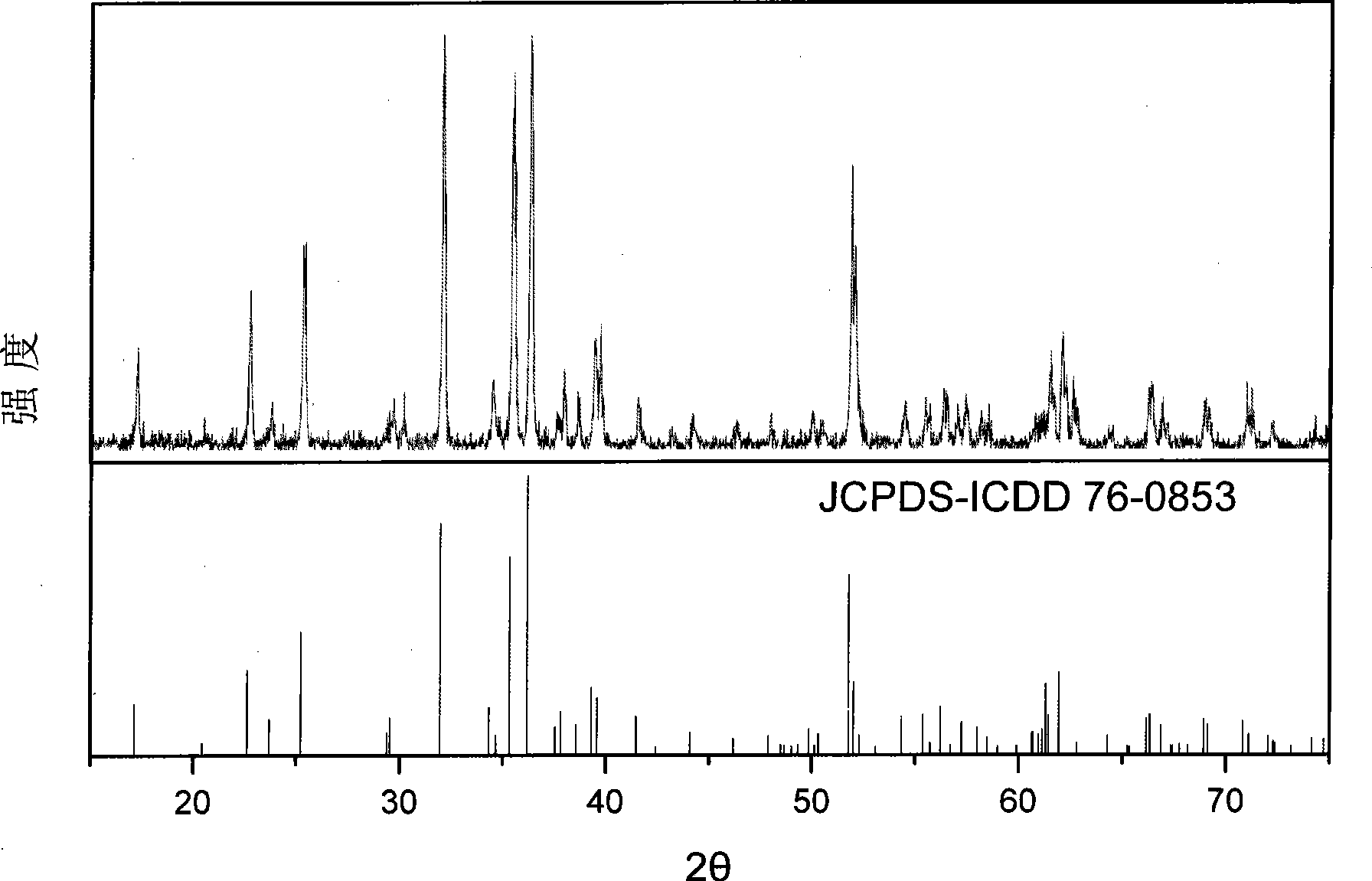
[0028] The powder X-ray diffraction experiment was carried out on the Rigaku D / MAX2200PC X-ray diffractometer produced by Shimadzu Corporation of Japan with the positive electrode material iron magnesium silicate for the rechargeable magnesium battery prepared above. The experimental conditions are as follows: copper target, X-ray wavelength 0.15406 nm, Ni filter; the light tube voltage used is 40kV, the current is 20mA, the scanning range is 15-75°, and the scanning speed is 4° min -1 .
[0029] The anode material for rechargeable magnesium batter...
Embodiment 2
[0033] 0.6g of KCl, 0.0968g of MgO, 0.4354g of FeC 2 o 4 2H 2 O and 0.1452g of SiO 2 After grinding for 30 minutes until uniform, vacuum-dry at 100°C for 12 hours, then heat-treat at 350°C for 2 hours under the protective atmosphere of argon, then heat-treat at 1000°C for 6 hours, and finally cool naturally to room temperature to obtain Magnesium iron magnesium silicate is used as the positive electrode material for magnesium-charged batteries.
[0034] The anode material for rechargeable magnesium batteries prepared above was detected for metal elements on an Iris Advantage 1000 inductively coupled plasma emission spectrometer produced by American Thermoelectric Corporation. The results showed that the molar ratio of Mg and Fe was 1:1.
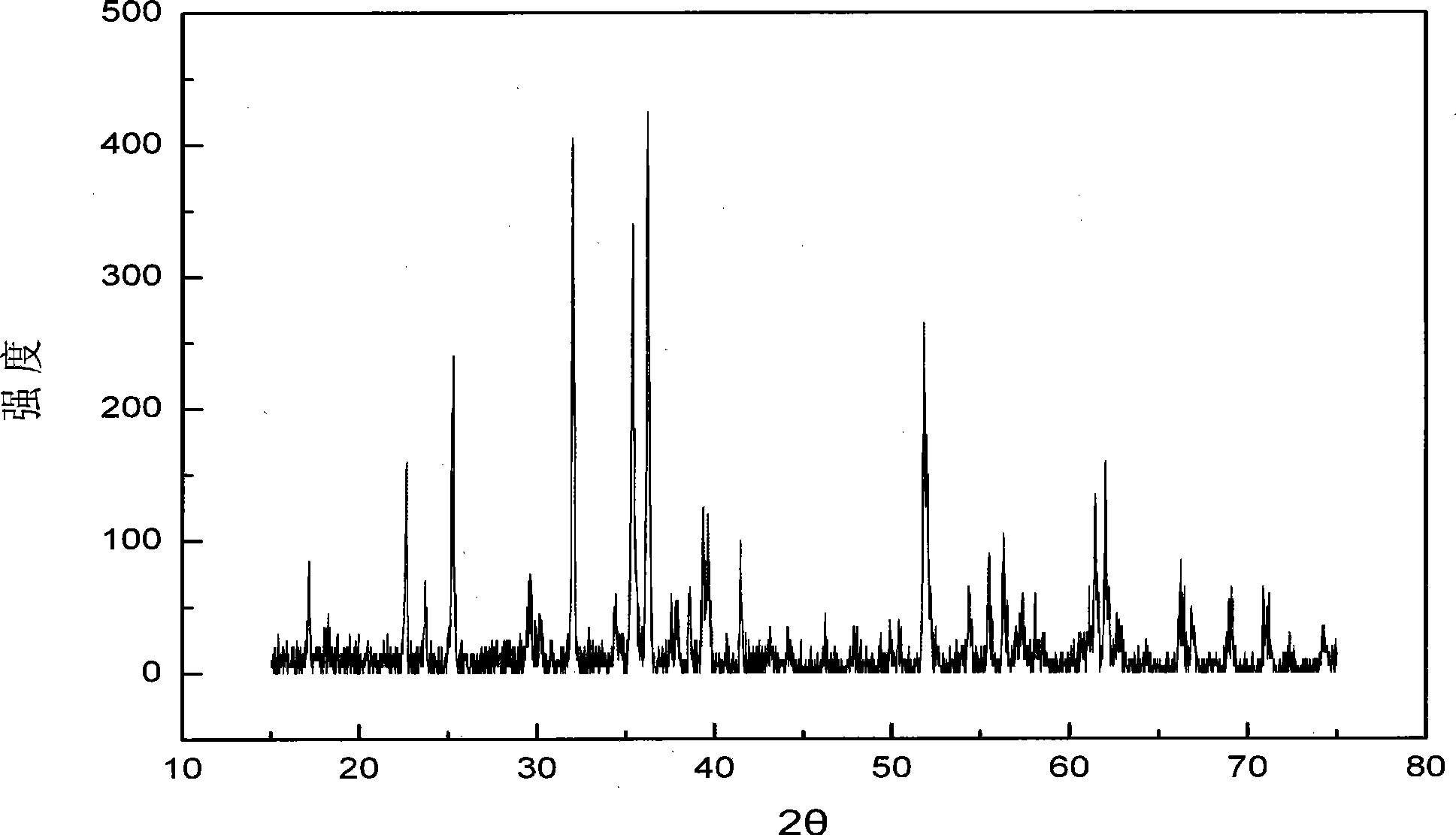
[0035] The powder X-ray diffraction experiment was carried out on the Rigaku D / MAX2200PC X-ray diffractometer produced by Shimadzu Corporation of Japan with the positive electrode material iron magnesium silicate for the rechargeable magne...
Embodiment 3
[0038] 0.6g of KCl, 0.0968g of MgO, 0.2878g of FeC 2 o 4 2H 2 O and 0.1202g of SiO 2 After grinding for 30 minutes until uniform, vacuum-dry at 100°C for 12 hours, then heat-treat at 350°C for 2 hours under a protective atmosphere of argon, then heat-treat at 1000°C for 6 hours, and finally cool naturally to room temperature to obtain a rechargeable Magnesium iron magnesium silicate is the cathode material for magnesium batteries.
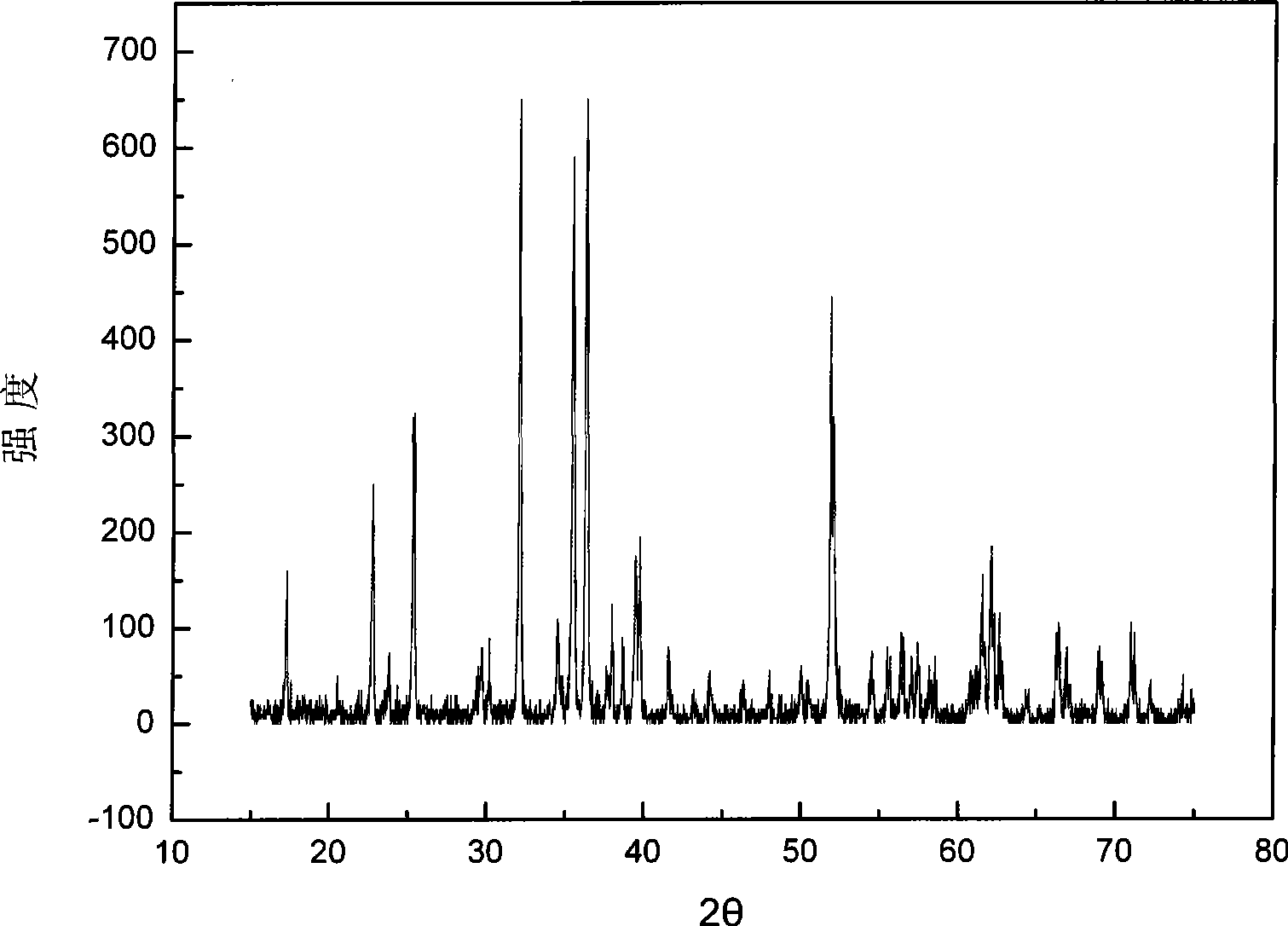
[0039] The powder X-ray diffraction experiment was carried out on the Rigaku D / MAX2200PC X-ray diffractometer produced by Shimadzu Corporation of Japan with the positive electrode material iron magnesium silicate for the rechargeable magnesium battery prepared above. The experimental conditions are as follows: copper target, X-ray wavelength 0.15406 nm, Ni filter; the light tube voltage used is 40kV, the current is 20mA, the scanning range is 15-75°, and the scanning speed is 4° min -1 .
[0040] The anode material for rechargeable magnesium b...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com