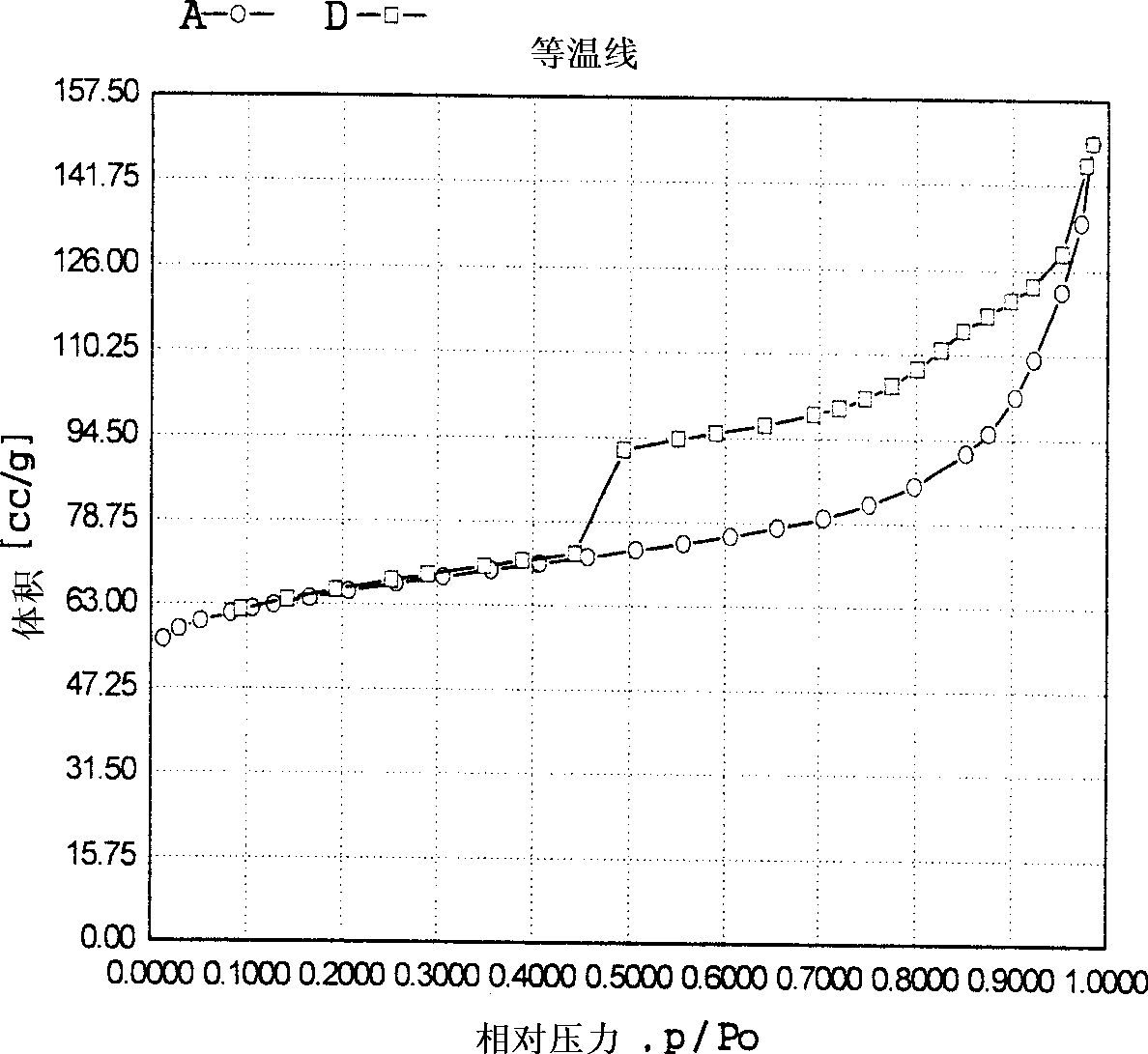
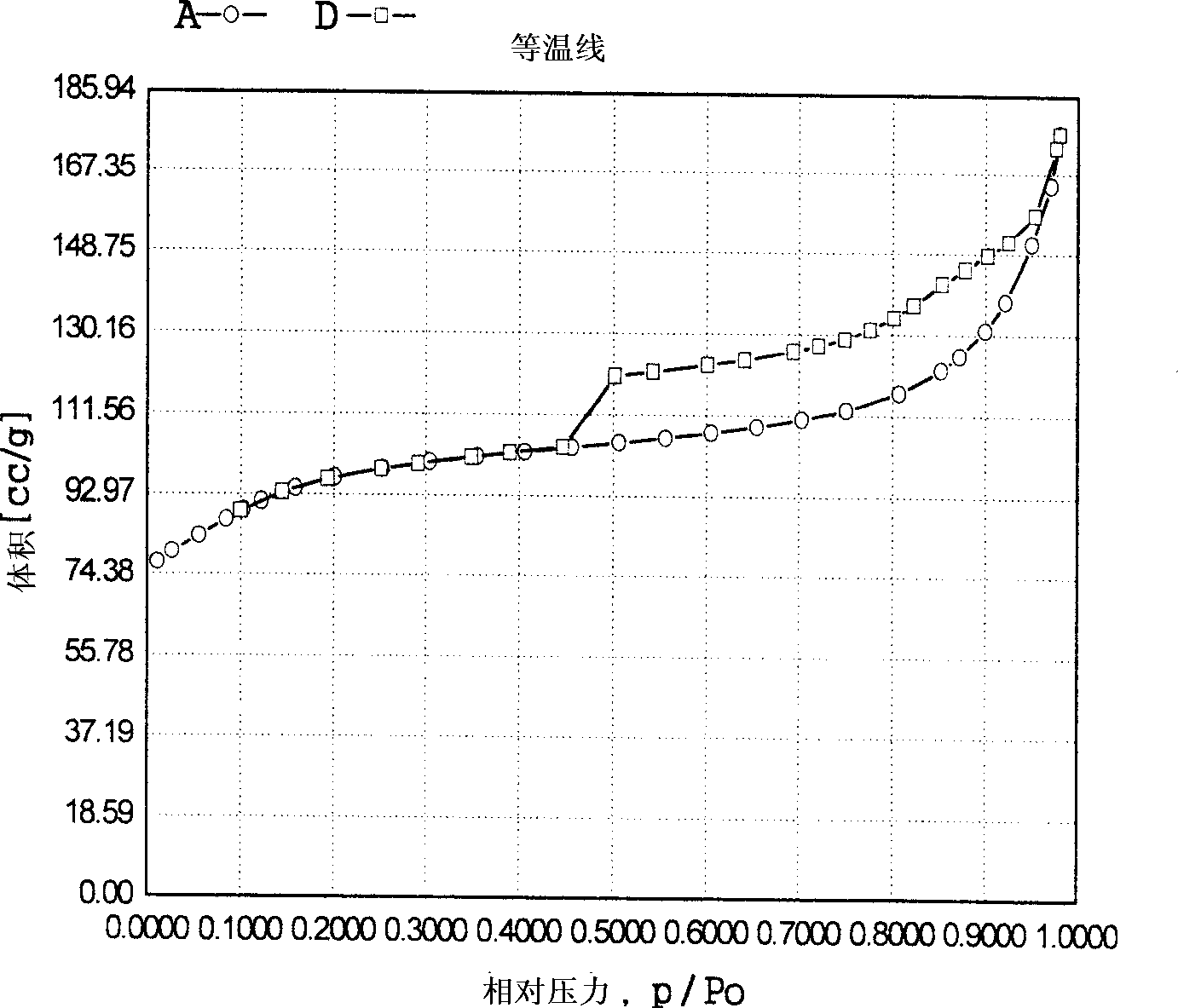
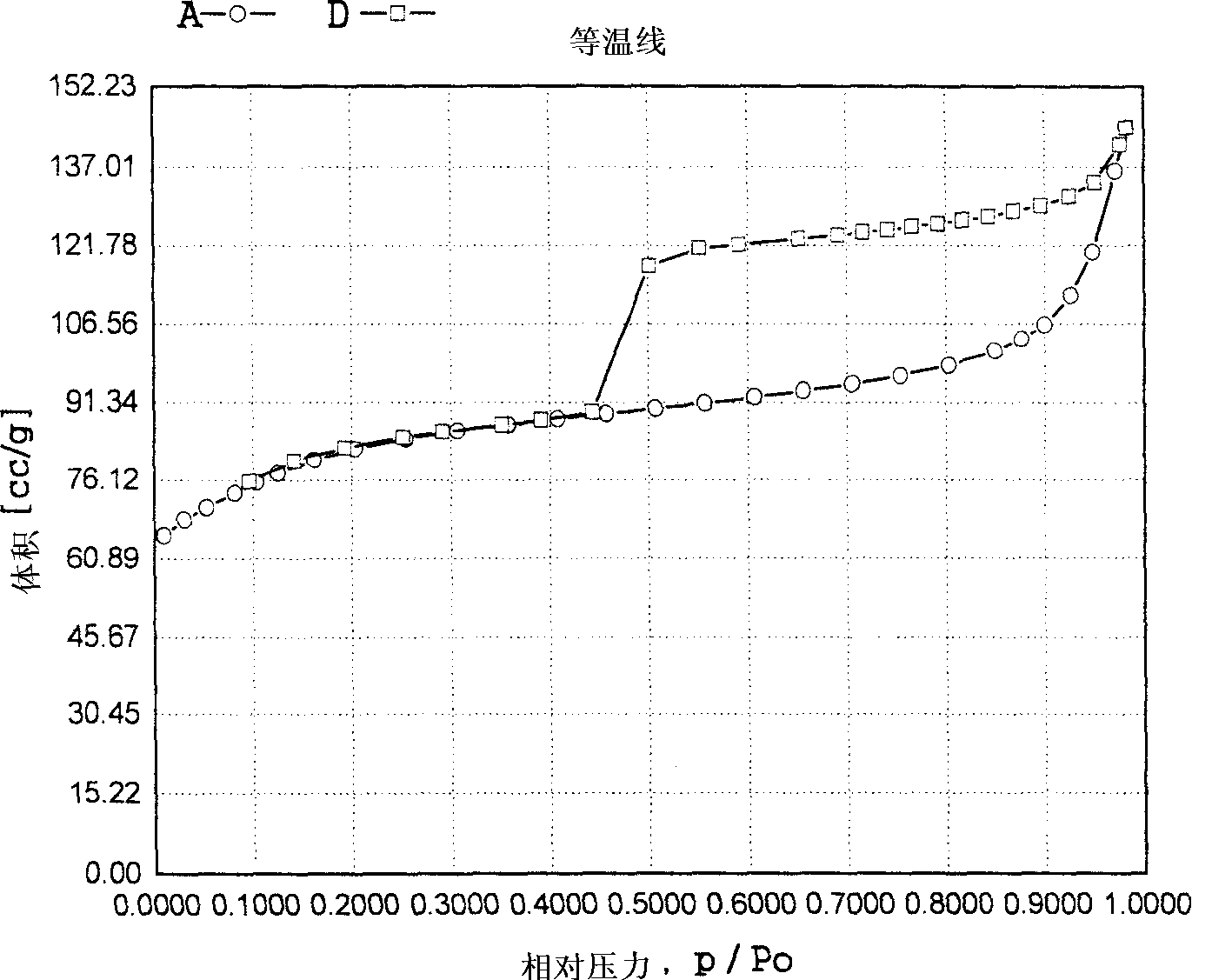
Propylene chloride epoxidation process in the presence of hydrogen and oxygen
A technology for epoxidation and propylene chloride, applied in chemical instruments and methods, organic chemistry, molecular sieve catalysts, etc., can solve problems such as harming the environment, short life, equipment corrosion, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069]Get 20 grams of titanium-silicon molecular sieve TS-1, concentration is 0.01g / ml (as palladium atom) ammonium nitrate palladium complex solution and appropriate amount of hydrazine hydrate and cetyltrimethylammonium bromide are added to tetrapropyl Aqueous solution of ammonium hydroxide (mass percentage concentration 10%) is stirred and mixed uniformly, wherein titanium silicon molecular sieve (gram): hexadecyltrimethylammonium bromide (mole): tetrapropyl ammonium hydroxide (mole): hydrated Hydrazine (mol): ammonium nitrate palladium complex (g, calculated as palladium): water (mol)=100:0.005:0.5:3.0:2.0:1000. Then put it into a stainless steel sealed reaction kettle, hydrothermally treat it at a temperature of 150°C and autogenous pressure for 48 hours, filter the resultant, wash with water, dry it naturally, and continue drying at 180°C for 3 hours to obtain micropores containing precious metals Titanium silicon material A. After characterization, its composition can ...
Embodiment 2
[0071] Get 20 grams of titanium-silicon molecular sieve TS-1, a palladium chloride solution with a concentration of 0.01 g / ml (in terms of palladium atoms), and an appropriate amount of hydrazine hydrochloride and polypropylene to join in an aqueous solution of sodium hydroxide (mass percentage concentration 15%) and stir Mix well, wherein titanium silicon molecular sieve (gram): polypropylene (mol): sodium hydroxide (mol): hydrazine hydrochloride (mol): palladium chloride (gram, in palladium): water (mol) = 100: 0.9: 1.8:0.15:0.1:4600. Then put it into a stainless steel sealed reaction kettle, hydrothermally treat it at a temperature of 180°C and autogenous pressure for 24 hours, filter the resultant, wash with water, dry it naturally, and continue drying at 110°C for 3 hours to obtain micropores containing precious metals. Titanium silicon material B. After characterization, its composition can be expressed as 8TiO in the form of oxides 2 100SiO2 2 ·0.006PdO·0.008Pd, the ...
Embodiment 3
[0073] Tetraethyl orthosilicate, tetrabutyl titanate, palladium acetate solution and Tween 80 with a concentration of 0.01 g / ml (in terms of palladium atoms) were added to the aqueous solution of tetrapropylammonium hydroxide and butylenediamine (mass The percentage concentration is 10%) and stir and mix evenly, wherein the molar composition silicon source: titanium source: tetrapropylammonium hydroxide: butylenediamine: palladium source: protective agent: water = 100: 0.03: 0.5: 0.1: 0.05: 0.02:550, silicon source is SiO 2 In terms of titanium source as TiO 2 In terms of palladium source in terms of Pd. Then put it into a sealed reactor, hydrothermally treat it at a temperature of 120°C and autogenous pressure for 120 hours, take out the resultant, filter it, dry it, and roast it to obtain an intermediate crystalline material. Transfer the intermediate crystalline material to the above remaining filtrate, add an appropriate amount of hydrazine hydrate, and then conduct a hy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com