Synthesis of 1,4-diene-6-methylene steroids and midbody thereof
A technology of methylene steroid and synthesis method, applied in the directions of steroids, organic chemistry, etc., can solve the problems of high production cost, difficult impurities, difficult reaction, etc., and achieve the effects of low cost, easy source and cheap source.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
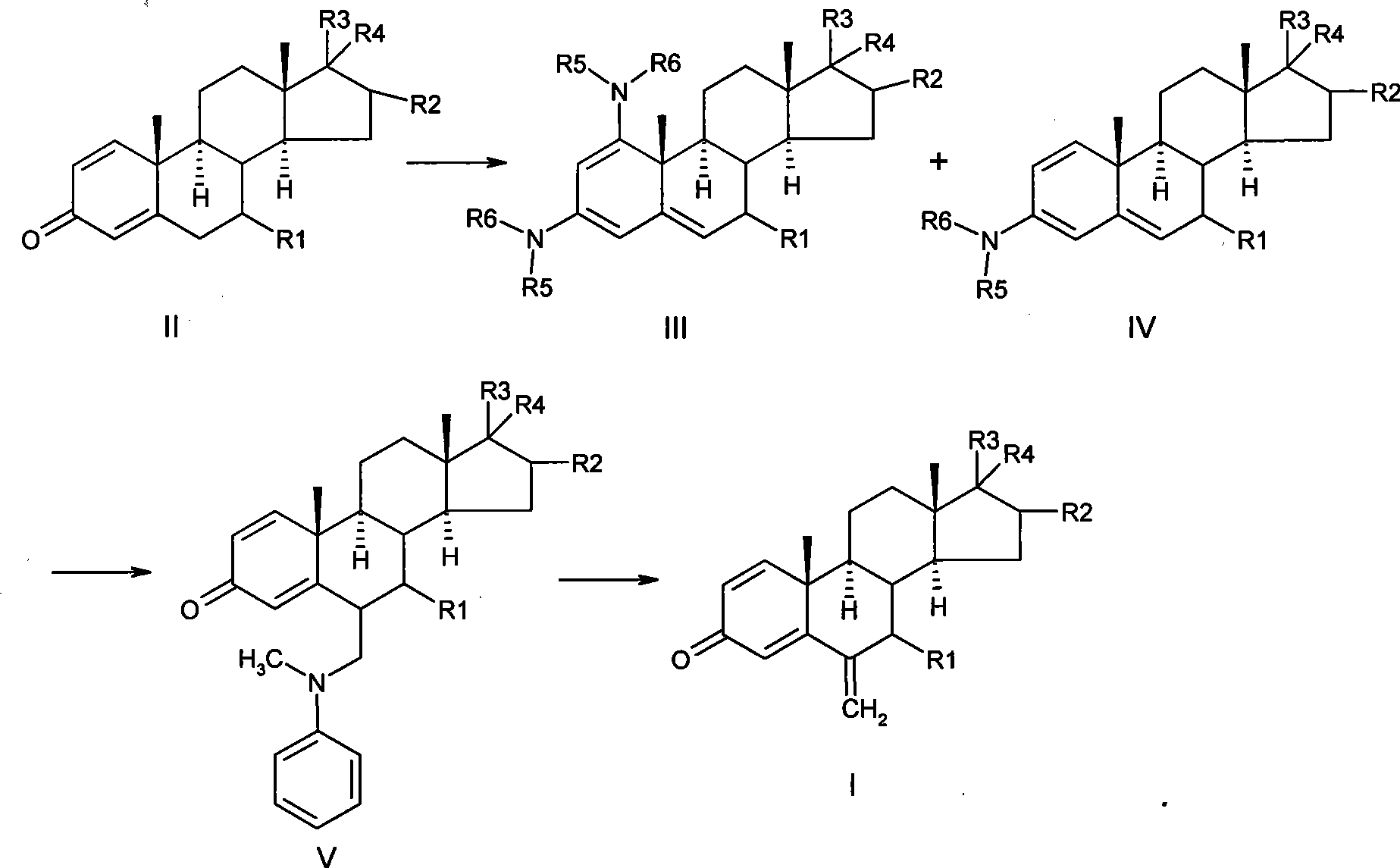
[0040] Preparation of 1,3-dipyrrolidine-androst-3,5-dien-17-one and 3-pyrrolidine-androst-1,3,5-trien-17-one
[0041] Under argon protection, dissolve 10.0 g of 1,4-diene-3,17-dione-androster in 100 ml of ethanol / methanol (95:5), then add 6 ml of tetrahydrofuran, 0.2 ml of glacial acetic acid, and 35.4 ml of Tetrahydropyrrole was heated to 40°C and stirred for 48 hours. After the reaction was completed, it was concentrated to dryness, and distilled twice with isopropyl ether 30ml×2. Then add 20ml of absolute ethanol, stir evenly, put it into the refrigerator for crystallization, and freeze for 24 hours. Filter, fully rinse with 15ml of frozen ethanol × 2, pump dry, and vacuum-dry at 40°C for 6 hours to obtain 11.7g of a yellow solid, which is 1,3-dipyrrolidine androst-3,5-dien-17-one and 3 - a mixture of pyrrolidine-androst-1,3,5-triene-17-one, the two are directly subjected to the next reaction without separation.
Embodiment 2
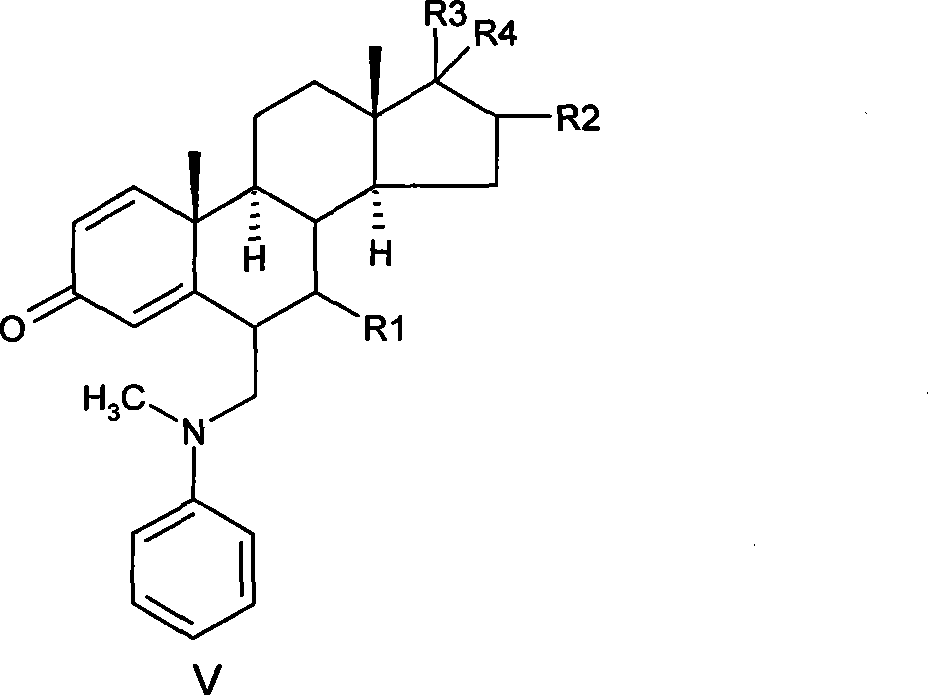
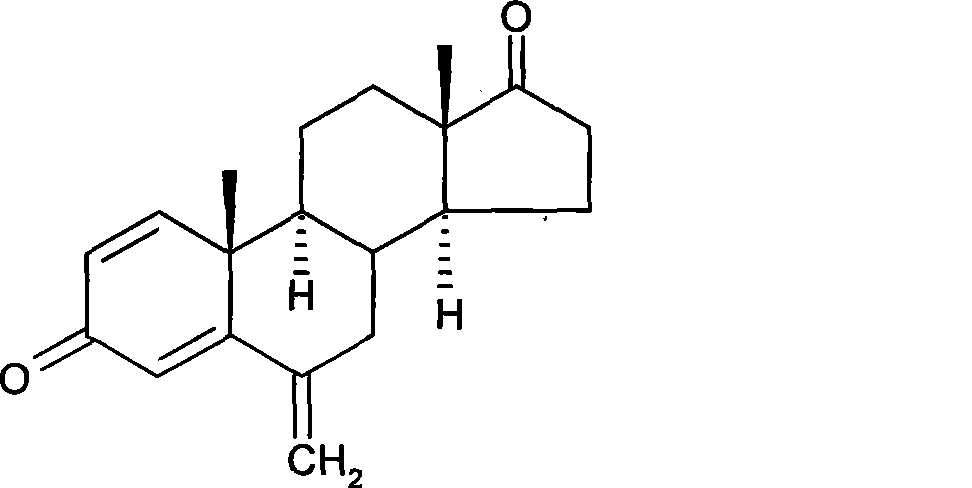
[0043] Preparation of 6-methylphenylamine methylene-androst-1,4-diene-3,17-dione
[0044] Under the protection of argon, 5.0 g of the solid obtained in Example 1 was suspended in 50 ml of absolute ethanol with stirring, and then 1.41 ml of N-methylaniline and 5.94 ml of 40% aqueous formaldehyde were added successively, and the reaction was stirred at 20° C. for 3.5 hours. After the reaction, concentrate to dryness at 40°C, dissolve the residue with 50ml of dichloromethane, wash 3 times with 50ml of 1% dilute sulfuric acid, combine the aqueous layers, back extract once with 50ml of dichloromethane, combine the organic layers, and wash with saturated bicarbonate Sodium solution (50ml×2) was washed until neutral, dried over anhydrous sodium sulfate, filtered, and concentrated to dryness at 40°C to obtain 4.4g of a yellow solid with a weight yield of 88%.
Embodiment 3
[0046] Preparation of 6-methylphenylamine methylene-androst-1,4-diene-3,17-dione
[0047] Under the protection of argon, 5.0 g of 1,3-dipyrrolidinandrost-3,5-dien-17-one made according to US3274176 was suspended in 50 ml of absolute ethanol with stirring, and then 1.41 ml of N -Methylaniline and 5.94ml of 40% formaldehyde aqueous solution were stirred and reacted at 15°C for 4 hours. The aftertreatment was the same as above to obtain 3.5 g of a yellow solid with a weight yield of 70%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com