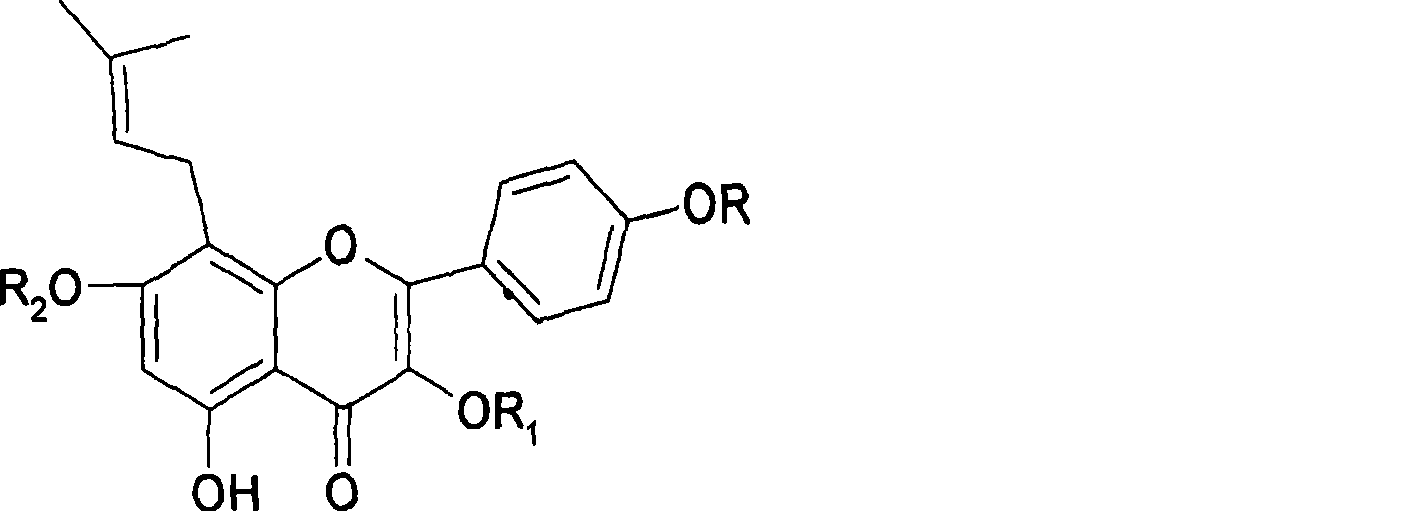
Uses of icaritin in preparing medicament for preventing and treating endotoxemia
A technology for endotoxemia and icariin, which is used in drug combinations, antidote, blood diseases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1 Effect of Icaritin on the Mortality of Mice After Endotoxin Challenge
[0019] Experimental drugs: icariin (Shanghai Ronghe Medicine, batch number: 20081578, HPLC purity 98%), dexamethasone (sigma, batch number: D4902), LPS (sigma, L2880).
[0020] Experimental animals: C57BL / 6J mice, half male and half male, 18-22g (Shanghai slack), observed in the observation room for 3 days before administration, the animals were randomly divided into groups, with an average of 6 mice per cage, and the room temperature was 20°C to 25°C. ℃, the relative humidity is 50% to 70%, and the light-dark cycle is 12h.
[0021] Experimental method: The experimental animals were randomly divided into 6 groups: blank group, model group, low-dose icariin group, middle-dose icariin group, high-dose icariin group, and dexamethasone control group. 12 only. Among them, 25mg / kg.d, 50mg / kg.d, 100mg / kg.d icariin, 5mg / kg. d Intragastric administration of dexamethasone for 3 consecutive days, ...
Embodiment 2
[0027] Example 2 In vitro experiment of the effect of icariin on endotoxin-related inflammation in mice
[0028] Experimental drug: with embodiment 1.
[0029] Cell line: mouse macrophage cell line RAW264.7 (purchased from Shanghai Cell Bank, Chinese Academy of Sciences).
[0030] experimental method:
[0031] (1) Culture and passage of RAW264.7 cells
[0032] Mouse macrophage RAW246.7 was added to high-glucose DMEM containing 10% fetal bovine serum, 100U / ml penicillin, and streptomycin, and placed in a 5% CO2, 37°C incubator to grow, and the cells grew to 70-80% Subculture after fusion, once every 2-3 days.
[0033] (2) RAW264.7 cell viability detection:
[0034] Adjust the cell suspension concentration to 1×10 5 / ml, add to 96-well culture plate, 100 μl per well, put in 37℃ 5% CO2 incubator and cultivate for 6h, suck out the old culture solution, add icariin (1ug / ml, 10ug / ml, 100ug / ml respectively) ), medium containing dexamethasone (5ug / ml), medium containing 0.1% DMS...
Embodiment 3
[0054] Example 3 In vivo experiment of the effect of icariin on endotoxin-related inflammation in mice
[0055] Experimental drug: with embodiment 1.
[0056] Experimental animal:: with embodiment 1.
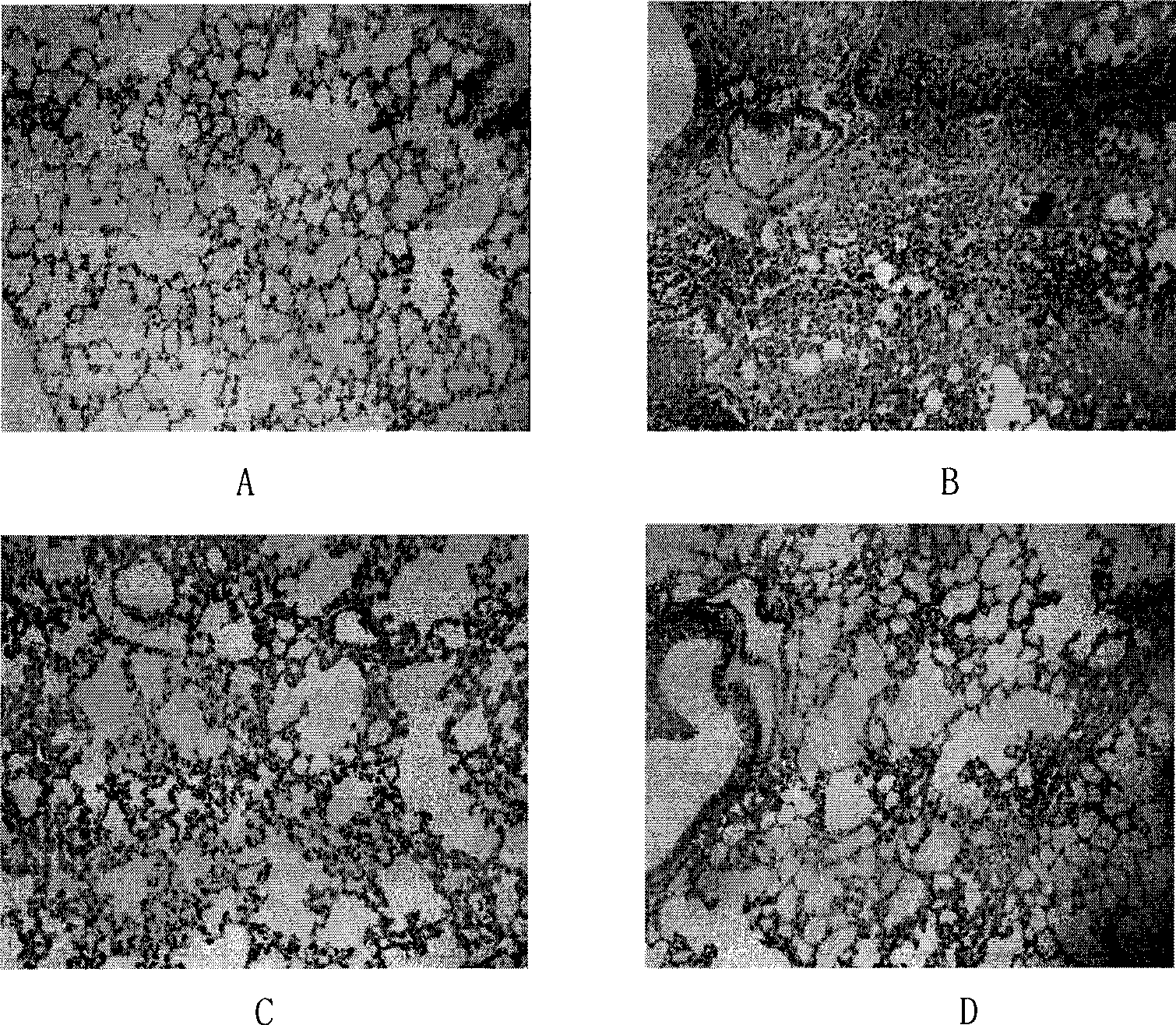
[0057] Animal modeling and drug intervention: Experimental animals were randomly divided into 4 groups: blank group, model group, treatment group and western medicine control group, 8 animals in each group; treatment group (icariin 25 mg / kg), western medicine control group (ground Symetasone 5mg / kg) was intragastrically administered with DMSO, and the blank group and the model group were intragastrically administered with DMSO of the same volume; 2 hours after intragastric administration, LPS1mg / kg was injected intraperitoneally, and the blank group was injected with normal saline. Under sodium anesthesia, blood was collected from the heart, and the lungs were separated.
[0058] Detection of TNF-α and IL-6: Let the whole blood stand at room temperature for more than 30 minute...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com