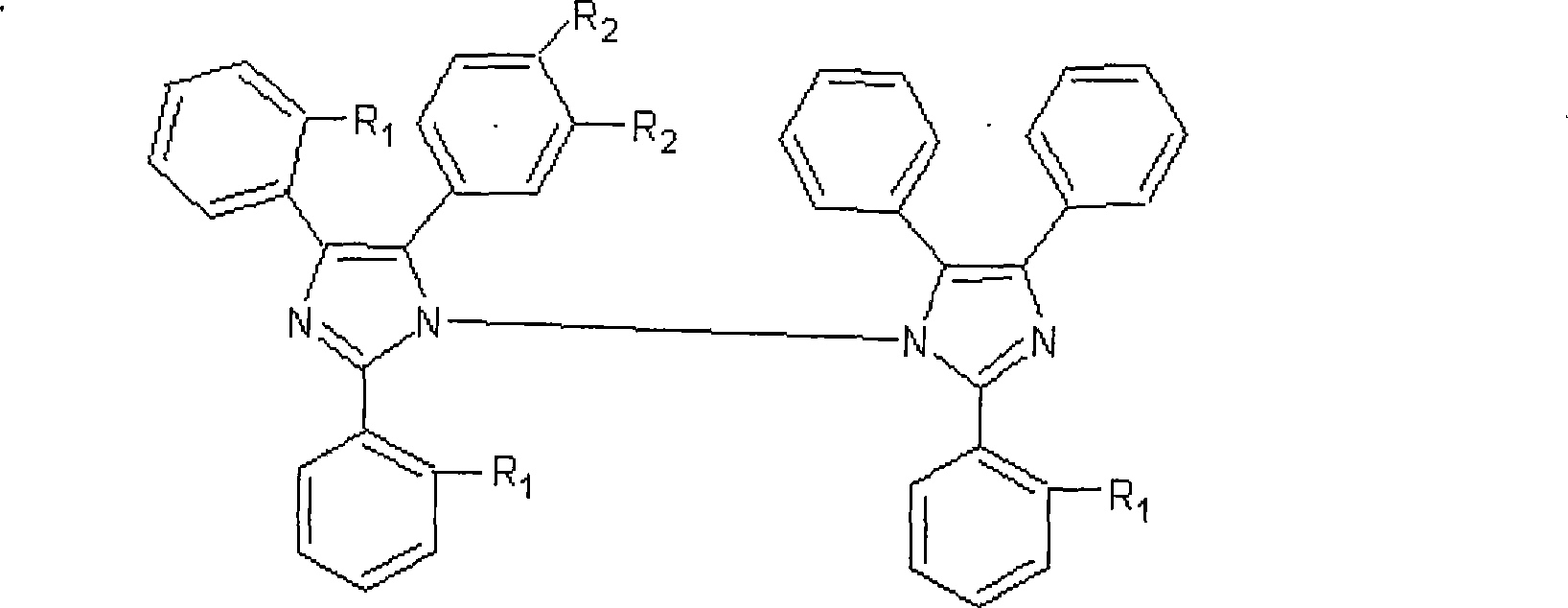
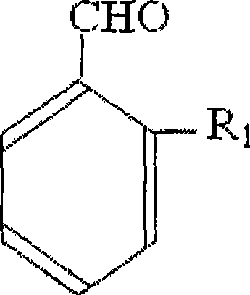
Photoinitiator contaning asymmetic hexaaryl bis imidazole and preparation method thereof
A technology of hexaarylbiimidazole and photoinitiator, which is applied in the field of photoinitiator and its preparation, can solve the problems of large usage, low photoinitiation activity, and low solubility, and achieve excellent solubility, high photosensitive performance, and reduce The effect of emissions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0026] A) Synthesis of Benzoin
[0027] The reaction was carried out in a one-liter four-neck flask equipped with a stirrer, reflux concentration equipment, and nitrogen protection. The reactant feed is shown in the table below:
[0028] Table 1 The synthesis feed list of benzoin
[0029] raw material Quantity (grams) Benzaldehyde 264 (2.5mols) Methanol 225ml water 65ml potassium cyanide 10
[0030] The reaction system was protected with nitrogen, and the reaction mixture was refluxed for 45 minutes, then cooled, and the four-neck flask was cooled with an ice bath to precipitate crystals. The precipitated crystals were filtered, washed with a mixture of 175 ml of methanol and 25 ml of water, washed with 1000 ml of water, and then recrystallized with ethanol. Obtain benzoin product 235g, yield>95%, melting point 134~136 ℃, product purity>98%.
[0031]
[0032] The structural formula of benzoin
[0033] B) Synthesis of 3,4-dimethox...
example 2
[0088] A) Synthesis of Benzoin
[0089] The reaction was carried out in a one-liter four-neck flask equipped with a stirrer, reflux concentration equipment, and nitrogen protection. The reactant feed is shown in the table below:
[0090] Table 11 Synthetic feed list of benzoin
[0091] raw material Quantity (grams) Benzaldehyde 264 (2.5mols) Methanol 225ml water 65ml potassium cyanide 10
[0092] The reaction system was protected with nitrogen, and the reaction mixture was refluxed for 45 minutes, then cooled, and the four-neck flask was cooled with an ice bath to precipitate crystals. The precipitated crystals were filtered, washed with a mixture of 175 ml of methanol and 25 ml of water, washed with 1000 ml of water, and then recrystallized with ethanol. Obtain benzoin product 235g, yield>95%, melting point 134~136 ℃, product purity>98%.
[0093] B) Synthesis of 3,4-diethoxy-2'-chloro-benzoin
[0094] Table 12 3,4-diethoxy-2'-chlor...
example 3
[0138] The produced mixture containing asymmetric hexaarylbiimidazole was applied to photocurable ink. The formula of photocurable ink is as follows:
[0139] Table 21 Formula table of photocurable ink
[0140]
[0141] The mixture formed by formula I can also be cured in visible light, has strong absorption of ultraviolet-visible light, and has high sensitivity; the mixture of formula II can only be cured under the irradiation of ultraviolet lamps, and has low sensitivity. The photoinitiator mixture containing the asymmetric hexaarylbiimidazole greatly improves the sensitivity of the photocurable ink and effectively saves energy consumption.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com