Cracking catalyst, process for preparation thereof, and process for catalytic cracking of hydrocarbon oil
A catalytic cracking and catalyst technology, applied in the direction of catalyst activation/preparation, catalytic cracking, physical/chemical process catalysts, etc., can solve the problems of FCC gasoline yield reduction, tar increase, etc., to reduce burden, high cracking activity, and reduce costs Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093]
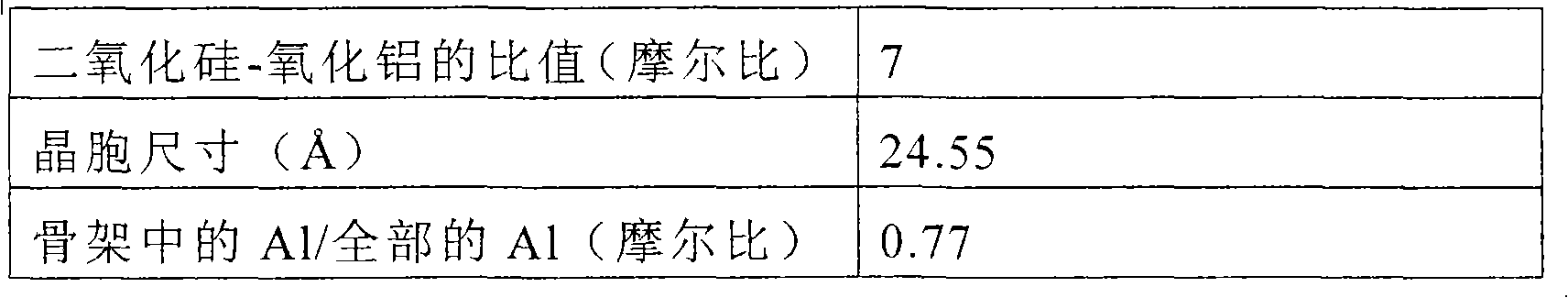
[0094] Catalyst A was prepared by the following procedure using the following ingredients: Ultrastable Y-type zeolite as a crystalline aluminosilicate having the properties shown in Table 1; silicic acid as a binder Sodium (JIS No.3 sodium silicate; SiO 2 content of 28.9 mass %); kaolinite as a clay mineral, and hydrated alumina (trade name: Pural SB; manufactured by Sasol Co., Ltd.) as a catalyst additive, which has a boehmite-like structure.
[0095] The solution prepared after mixing 138g of sodium silicate aqueous solution with distilled water was added dropwise in dilute sulfuric acid to obtain silica hydrosol (SiO 2 content is 10.2% by mass). On the other hand, distilled water was added to 76.0 g (on a dry basis) of Ultrastable Y-type zeolite having the properties shown in Table 1 to prepare a zeolite slurry. 73.4 g (dry basis) of kaolinite and 10.0 g (dry basis) of hydrated alumina were added to the silica hydrosol. These ingredients were mixed together, a...
Embodiment 2
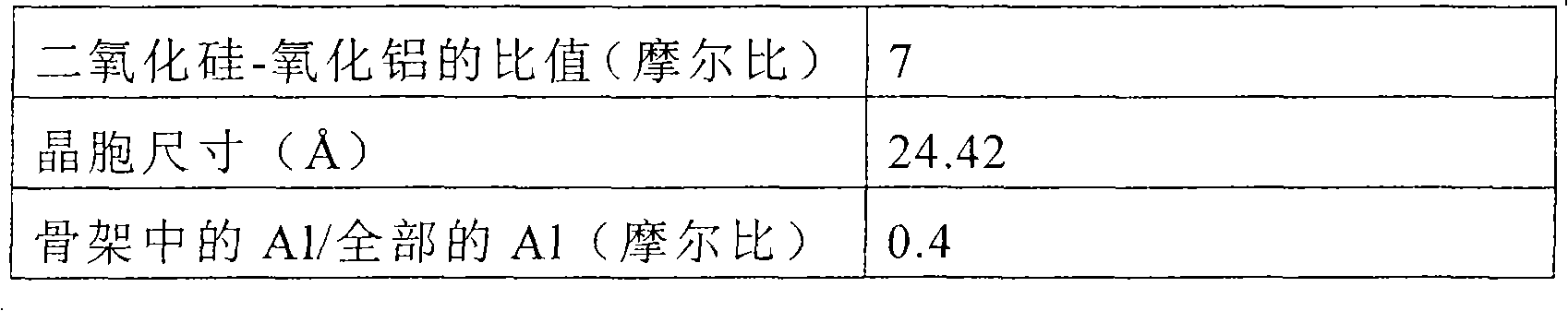
[0102] Ultrastable Y-type zeolite having the properties shown in Table 2 was used as the crystalline aluminosilicate.
[0103] [Table 2]
[0104]
[0105] Catalyst B was prepared according to the same procedure as in Example 1, except that the crystalline aluminosilicate described in Table 2 was used, and the amount of mixed kaolinite was changed to 73.0 g (on a dry basis), and The catalyst was ion-exchanged with lanthanum so that the content of lanthanum oxide was 0.5% by mass in a dry state.
[0106] The same procedure as in Example 1 was used to detect the xenon adsorption amount of catalyst B. Calculated from the results, the xenon adsorption capacity (N) is 2.46×10 under the condition that the xenon adsorption pressure is 650 Torr 20 molecules / gram of catalyst.
Embodiment 3
[0108] Ultrastable Y-type zeolite having the properties shown in Table 2 was used as the crystalline aluminosilicate.
[0109] Catalyst C is prepared according to the same procedure as in Example 1, and the difference is that the amount of ultrastable Y-type zeolite is changed from 76.0g (in dry state) to 80.0g (in dry state), and the amount of kaolinite is changed to 80.0g (in dry state). The amount was changed from 73.4 g (in dry state) to 67.6 g (in dry state), and the catalyst was ion-exchanged with lanthanum so that the content of lanthanum oxide was 1.2 mass % in dry state.
[0110] The xenon adsorption amount of catalyst C was detected by the same procedure as in Example 1. Calculated from the results, the xenon adsorption capacity (N) is 2.35×10 under the condition that the xenon adsorption pressure is 650 Torr 20 molecules / gram of catalyst.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| aperture size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com