Method for preparing semi-conductor luminescent material manganese-doped zinc sulfide nano powder
A technology of fluorescent materials and semiconductors, which is applied in the field of preparation of semiconductor fluorescent materials doped with manganese and zinc sulfide nanopowder, which can solve the problems of repeated grinding, long production cycle, and many crystal defects, and achieve excellent semiconductor characteristics, fluorescent performance, and reaction temperature And the effect of low energy consumption and simple production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
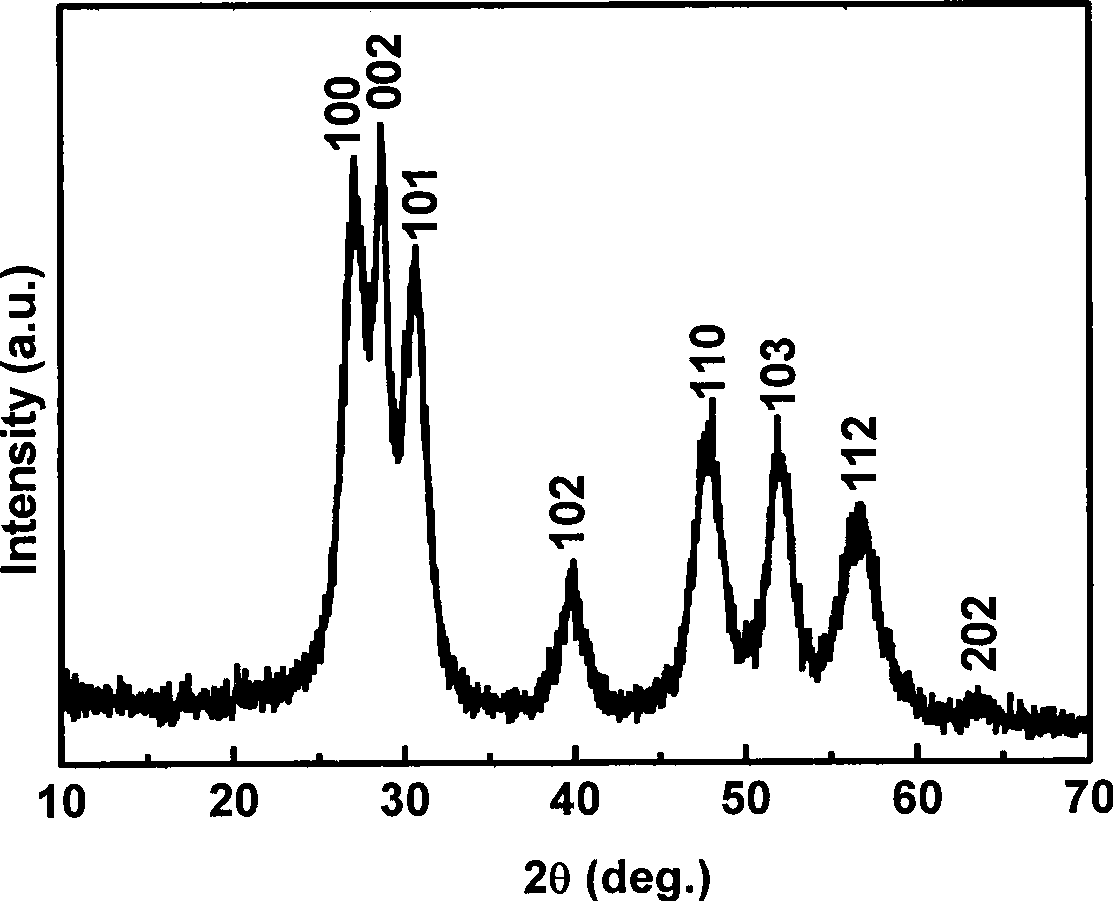
[0020] 1. Weigh 0.1mmol manganese acetate (Mn(CH 3 COO) 2 2H 2 O) and 9.9 mmol zinc acetate (Zn(CH 3 COO) 2 2H 2 O) powder; mix the two solid powders, add water to dissolve, and make a 200mL solution. 2. Weigh 20mmol sodium diethyldithiocarbamate (Na-DDTC) powder, add water to dissolve, and make 200mL solution. 3. Mix the above two solutions under stirring at room temperature, and keep stirring at room temperature for 2 hours, then filter the resulting precipitate to obtain the precursor Zn 0.99 mn 0.01 -(DDTC) 2 . 4. Weigh 1.0g precursor Zn 0.99 mn 0.01 -(DDTC) 2 Put it in a crucible, put it in a muffle furnace, heat it at 300°C for 3 hours, and then cool it down to room temperature naturally to get the hexagonal Zn 0.99 mn 0.01 S nano powder. Such as figure 1 , figure 2 As shown, the German Bruker AXS D8 ADVANCE X-ray powder diffractometer (Cu K α radiation, ) to determine the crystal phase of the prepared material; the shape and size of the product were ...
Embodiment 2
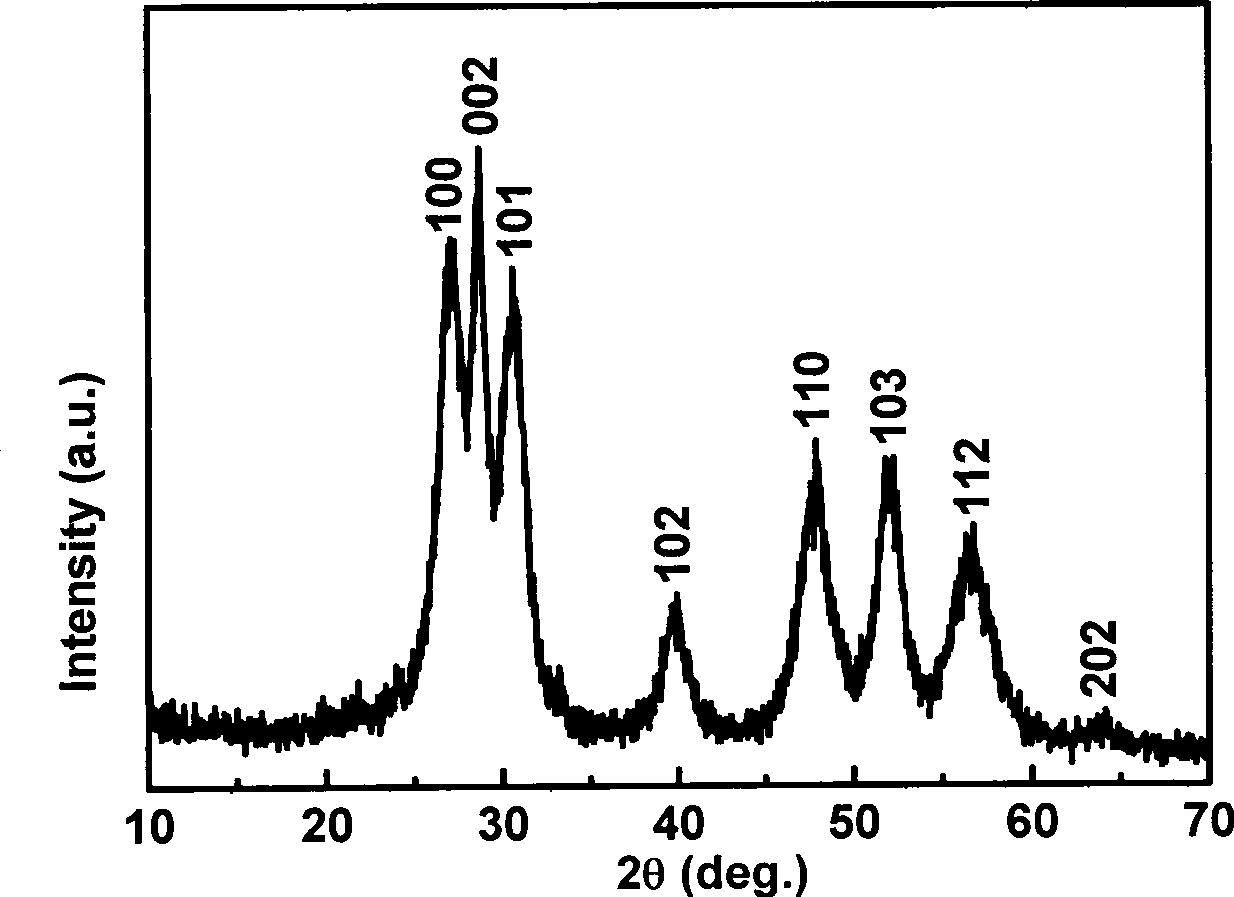
[0025] 1. Weigh 0.3mmol manganese acetate (Mn(CH 3 COO) 2 2H 2 O) and 9.7mmol zinc acetate (Zn(CH 3 COO) 2 2H 2 O) powder; mix the two solid powders, add water to dissolve, and make a 200mL solution. 2. Weigh 20mmol sodium diethyldithiocarbamate (Na-DDTC) powder, add water to dissolve, and make 200mL solution. 3. Mix the above two solutions under stirring at room temperature, and keep stirring at room temperature for 2 hours, then filter the resulting precipitate to obtain the precursor Zn 0.97 mn 0.03 -(DDTC) 2 . 4. Weigh 1.0g precursor Zn 0.97 mn 0.03 -(DDTC) 2 Put it in a crucible, put it in a muffle furnace, heat it at 300°C for 3 hours, and then cool it down to room temperature naturally to get the hexagonal Zn 0.97 mn 0.03 S nano powder. Such as image 3 , Figure 4 As shown, the German Bruker AXS D8 ADVANCE X-ray powder diffractometer (Cu K α radiation, ) to determine the crystal structure of the prepared material; the shape and size of the product we...
Embodiment 3
[0030] 1. Weigh 0.5mmol manganese acetate (Mn(CH 3 COO) 2 2H 2O) and 9.5mmol zinc acetate (Zn(CH 3 COO) 2 2H 2 O) powder; mix the two solid powders, add water to dissolve, and make a 200mL solution. 2. Weigh 20mmol sodium diethyldithiocarbamate (Na-DDTC) powder, add water to dissolve, and make 200mL solution. 3. Mix the above two solutions under stirring at room temperature, and keep stirring at room temperature for 2 hours, then filter the resulting precipitate to obtain the precursor Zn 0.95 mn 0.05 -(DDTC) 2 . 4. Weigh 1.0g precursor Zn 0.95 mn 0.05 -(DDTC) 2 Put it in a crucible, put it in a muffle furnace, heat it at 300°C for 3 hours, and then cool it down to room temperature naturally to get the hexagonal Zn 0.95 mn 0.05 S nano powder. Such as Figure 5 , Image 6 As shown, the German Bruker AXS D8 ADVANCE X-ray powder diffractometer (Cu K α radiation, ) to determine the crystal structure of the prepared material; the shape and size of the product wer...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com