Method for preparing nitrogen-containing onium salt compounds
A nitrogen compound and compound technology are applied in the field of preparation of nitrogen-containing onium salt compounds, which can solve the problems of high toxicity of halogenated hydrocarbons, insufficient variety of nitrogen-containing onium salt compounds, etc., and achieve low toxicity, simple method and wide source of raw materials. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
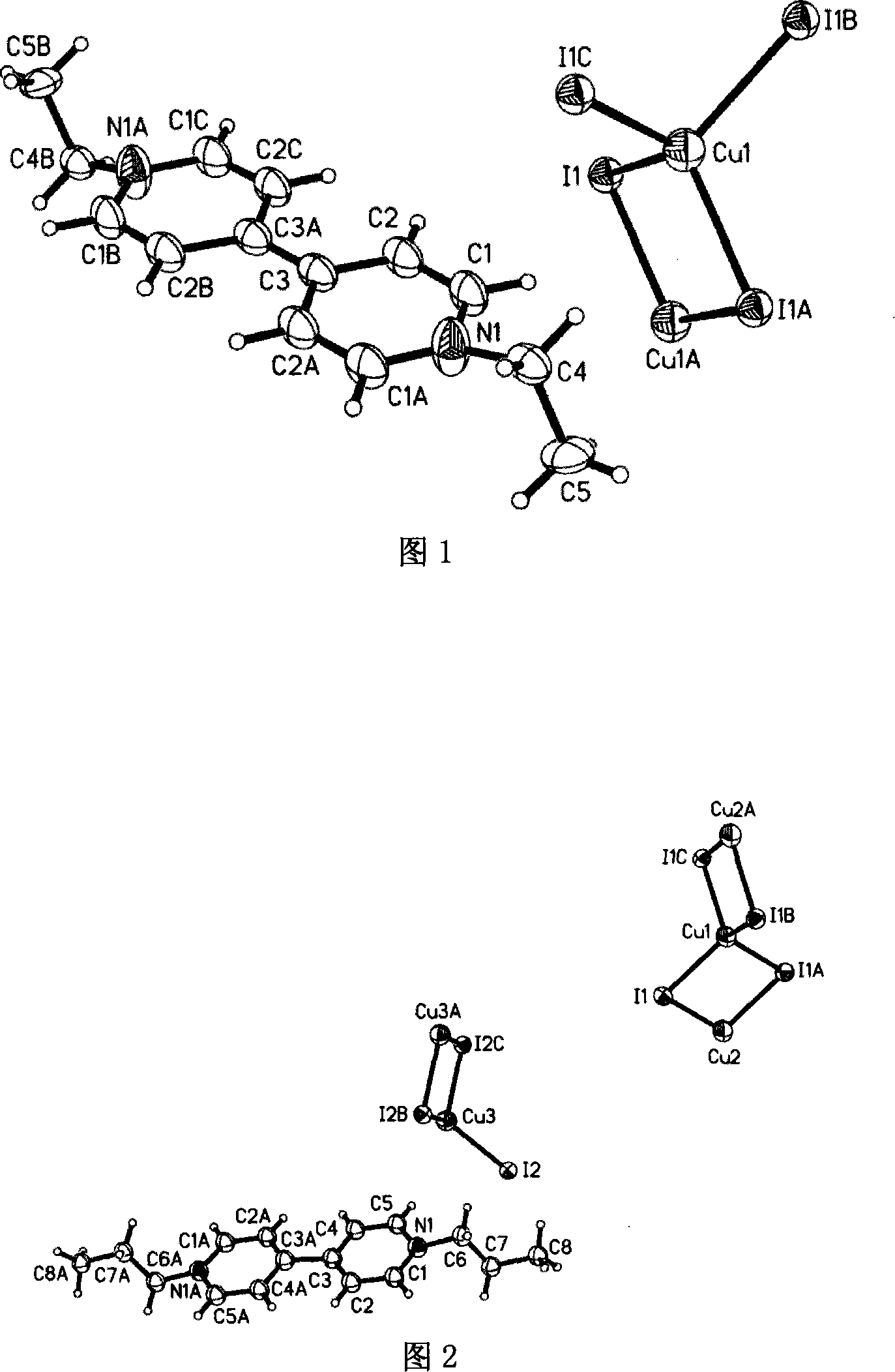
[0037] Embodiment one: referring to accompanying drawing 1, a kind of preparation method of nitrogen-containing onium salt compound comprises the following steps:
[0038] (1) Add 0.1 mmol of cuprous iodide (CuI), 0.1 mmol of iodine (I 2 ), 0.1 mmol 4,4'-bipyridine and 1 milliliter of ethanol are reaction initiators, mixed in 1 milliliter of acetonitrile, and reacted for 70 hours under solvothermal reaction conditions;
[0039] (2) Then, the temperature is gradually lowered at a rate of 5°C per 100 minutes to obtain black needle-like crystals with a single crystal structure {[C 14 h 18 N 2 ][Cu 2 I 4 ]} n As shown in Figure 1, the ethyl group on ethanol is transferred to the N of 4,4′-bipyridyl to form an onium cation [C 14 h 18 N 2 ] 2+ , the polyanion is one-dimensional (Cu 2 I 4 ) n 2n- chain.
[0040] (3) Pour acetonitrile into the precipitated mixture, stir, stop stirring, and pour it when the product crystals precipitate and the impurities do not precipitate,...
Embodiment 2
[0042] Embodiment two: referring to accompanying drawing 2, a kind of preparation method of nitrogen-containing onium salt compound comprises the following steps:
[0043] (1) 0.05 mmol of cuprous iodide, 0.05 mmol of iodine, 0.05 mmol of 4,4'-dipyridine and 0.5 milliliter of n-propanol are used as reaction starting materials, mixed in 1.5 milliliters of acetonitrile, and reacted in solvothermal Reaction under the conditions for 70 hours;
[0044] (2) Then gradually lower the temperature gradually at a rate of 5°C every 65 minutes to obtain black needle-like crystals with a single crystal structure {[C 16 h 22 N 2 ][Cu 2 I 4 ]} n As shown in Figure 2, the propyl group on n-propanol is transferred to the N of 4,4′-bipyridine to form an onium cation [C 16 h 22 N 2 ] 2+ , the polyanion is one-dimensional [Cu 2 I 4 ] n 2n- chain.
[0045] (3) The separation and purification process is the same as step (3) of Example 1. Yield: 83.11%.
[0046] (4) Elemental analysis...
Embodiment 3
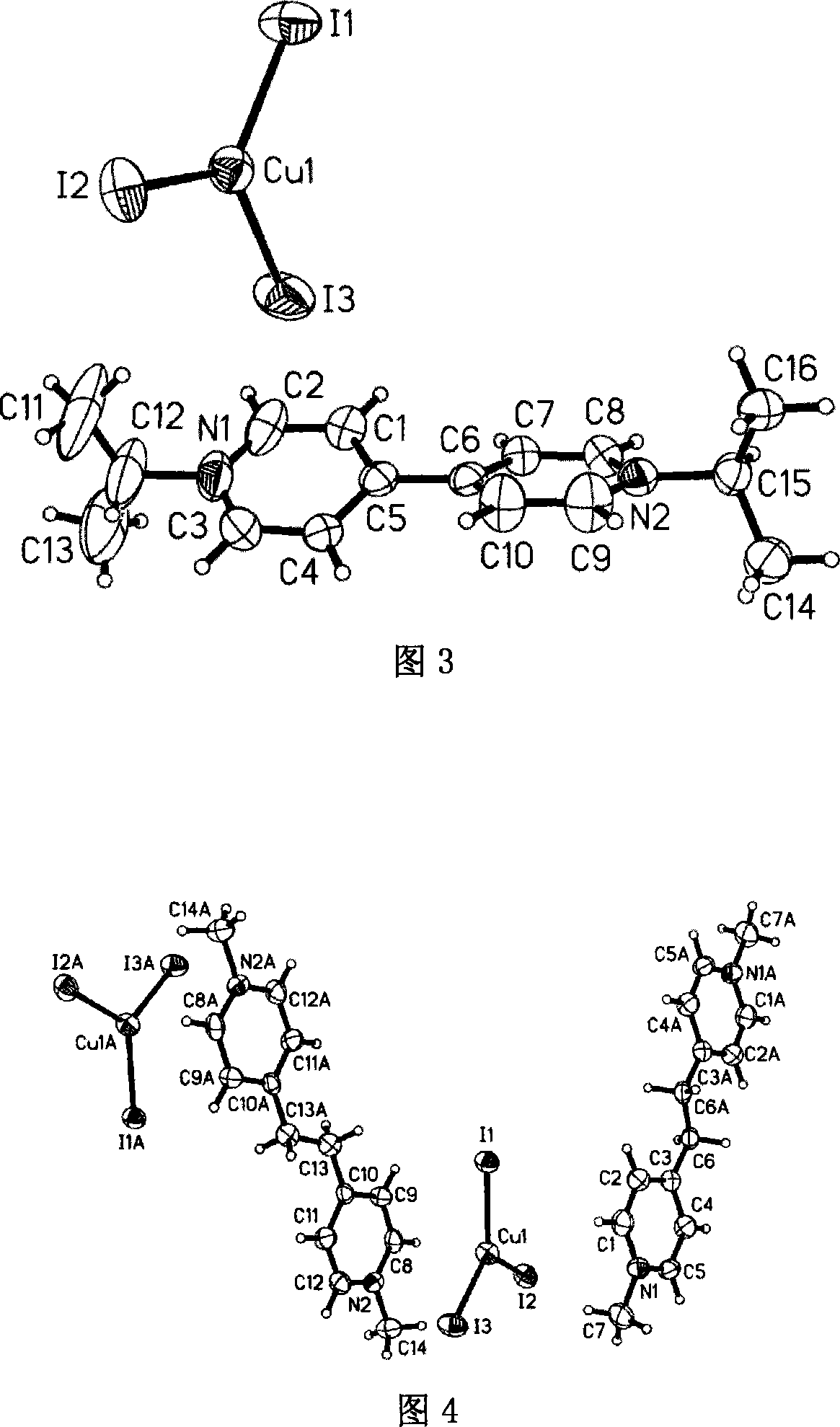
[0047] Embodiment three: referring to accompanying drawing 3, a kind of preparation method of nitrogen-containing onium salt compound comprises the following steps:
[0048] (1) 0.1 mmol of cuprous iodide, 0.1 mmol of iodine, 0.1 mmol of 4,4'-bipyridine and 1 ml of isopropanol were used as reaction starting materials, mixed in 1 ml of acetonitrile, and reacted in solvothermal Reaction under the conditions for 70 hours;
[0049] (2) Then gradually lower the temperature at a rate of 5°C every 100 minutes to obtain dark brown flaky crystals with a single crystal structure [C 16 h 22 N 2 ][CuI 3 ] As shown in Figure 3, the isopropyl group on the isopropanol is transferred to the N of 4,4'-bipyridine to form an onium cation [C 16 h 22 N 2 ] 2+ , with anion [CuI 3 ] 2- .
[0050] (3) The separation and purification process is the same as step (3) of Example 1. Yield: 79.45%.
[0051] (4) Elemental analysis: theoretical value (C 16 h 22 N 2 CuI 3 ): C, 27.99; H, 3.23;...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com