Expression of high temperature resistant xylanase gene in Kluyveromyces lactis
A technology of Kluyveromyces and xylanase, which is applied in the field of secretion and expression in Kluyveromyces lactis, can solve the problems of increased cost of separation and purification, achieve the effect of reducing the cost of separation and purification and improving expression efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
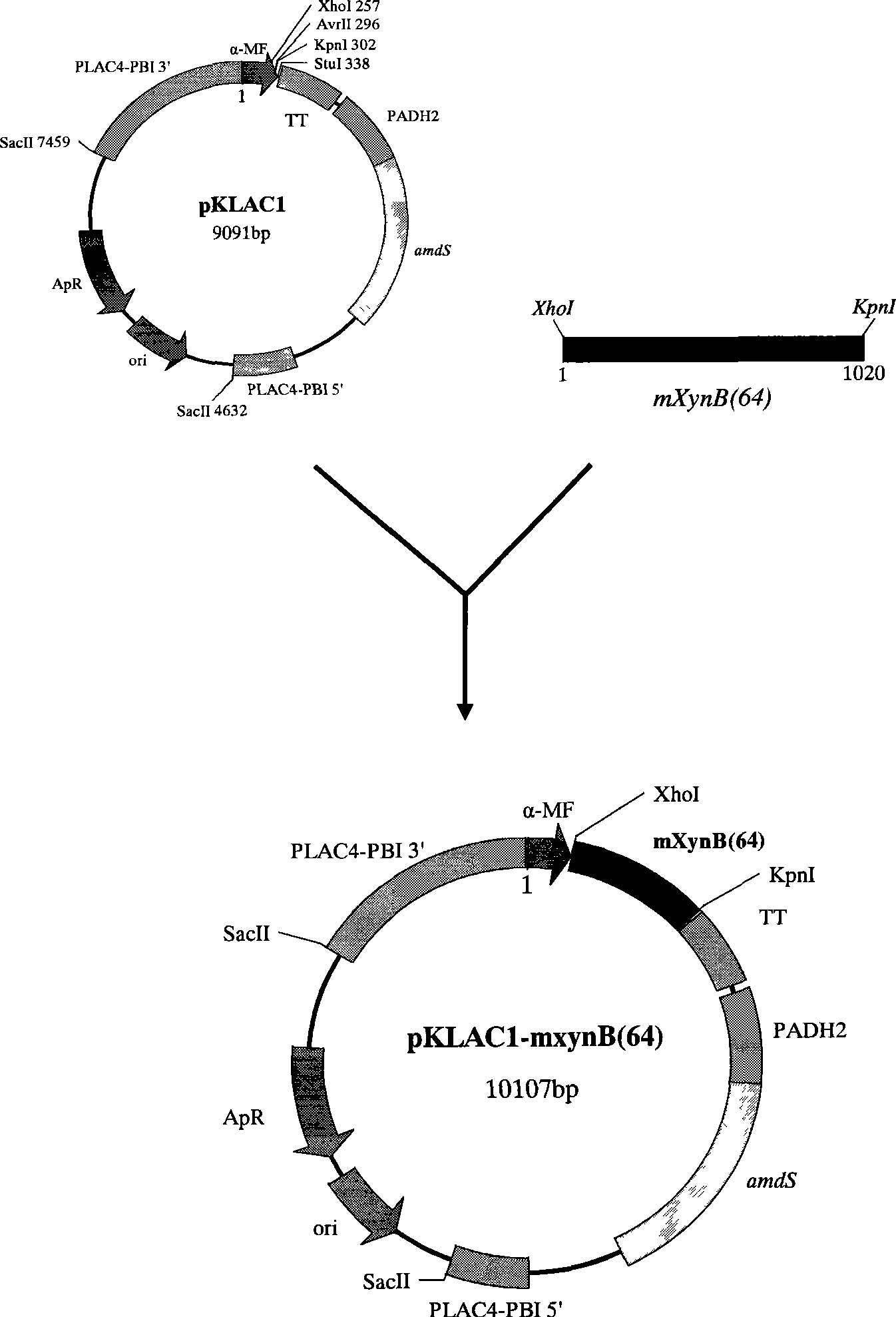
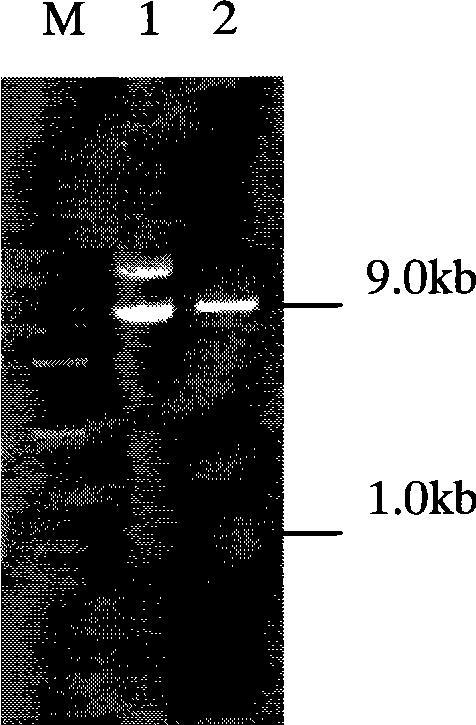
[0024] Example 1, Kluyveromyces lactis expression plasmid pKLAC1-mxynB (64) build
[0025] 1.1 Primer design
[0026] mxynB according to GenBank (64) Based on the sequence of the gene (GenBank accession number is AY340789) and the characteristics of the multiple cloning site of Kluyveromyces lactis expression vector pKLAC1, the following pair of primers were designed and synthesized:
[0027] PX1: FW: 5'-CCC ctcgag AAAAGAATGAAAATATTACCTTC-3'
[0028] PX2: REV: 5'-TTT ggtacc TCATTTTCTTTCTTCTATCTTTTTCT-3'
[0029] The two ends of PX1 and PX2 are designed with XhoI and KpnI restriction sites respectively (see the lowercase and underlined part in the above sequence)
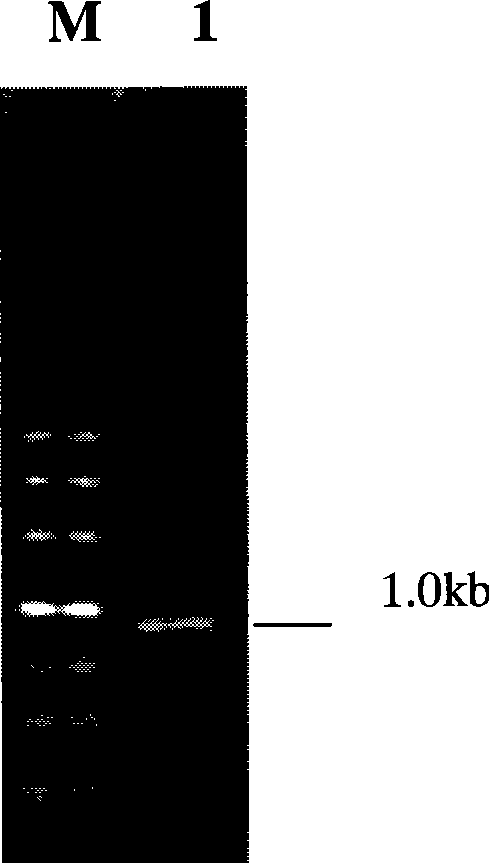
[0030] 1.2 Thermostable xylanase gene mxynB (64) PCR amplification
[0031] Using PX1 and PX2 primers, extract the genomic DNA of Thermotoga maritima, MSB8 (Thermotoga maritima, MSB8) as a template, (Thermotoga maritima, MSB8 genome registration number in GenBank: NC 000853, MSB8 bacterial strain can be fou...
Embodiment 2
[0041] Embodiment 2 thermostable xylanase gene (mxynB (64) ) secretory expression in Kluyveromyces lactis GG799
[0042] 2.1 Preparation of electroporation competent cells of Kluyveromyces lactis GG799 [purchased from New England BioLabs Inc. (NEB)] and electroporation transformation
[0043] (1) Pick a fresh single colony in 5ml YPD liquid medium (yeast powder 1%, peptone 2%, glucose 2%), and culture overnight at 30°C and 250rpm.
[0044] (2) Inoculate 100ml of freshly prepared YPD liquid at room temperature with an inoculation volume of 0.1% by volume
[0045] culture medium at 30°C, 250rpm to OD 600 0.4 to 0.5.
[0046] (3) Centrifuge at 3000 rpm for 5 minutes at 4°C to collect the cells;
[0047] (4) Wash the cells once with 250ml ice-cold sterile water;
[0048] (5) Wash the cells once with 50 ml of ice-cold sterile electroporation buffer EB (g / L): 92.4 sucrose, 2.03 magnesium chloride, 1 L of 10 mmol / L pH7.5 MES buffer;
[0049] (6) Resuspend the cells in 50ml ice-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com