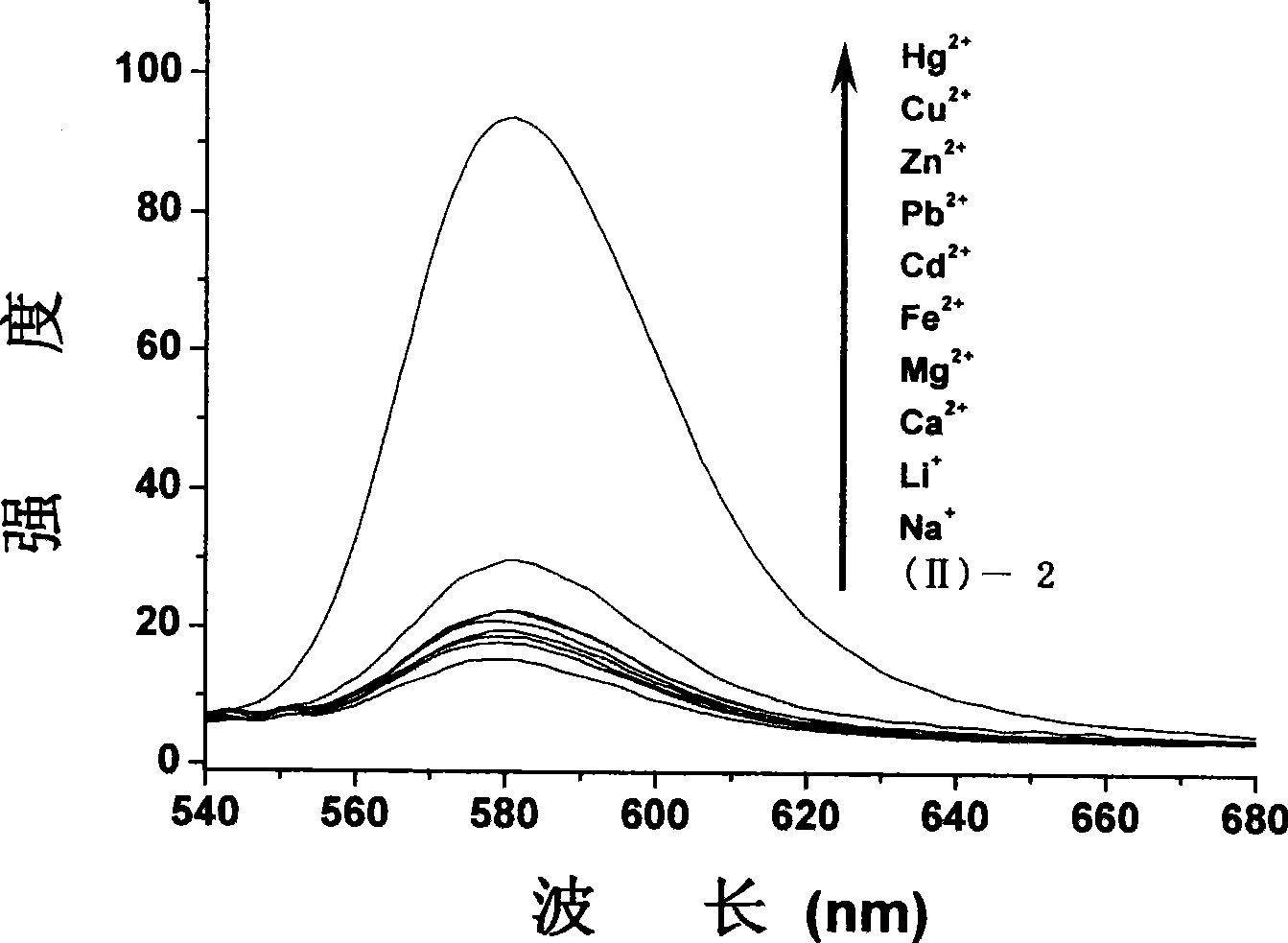
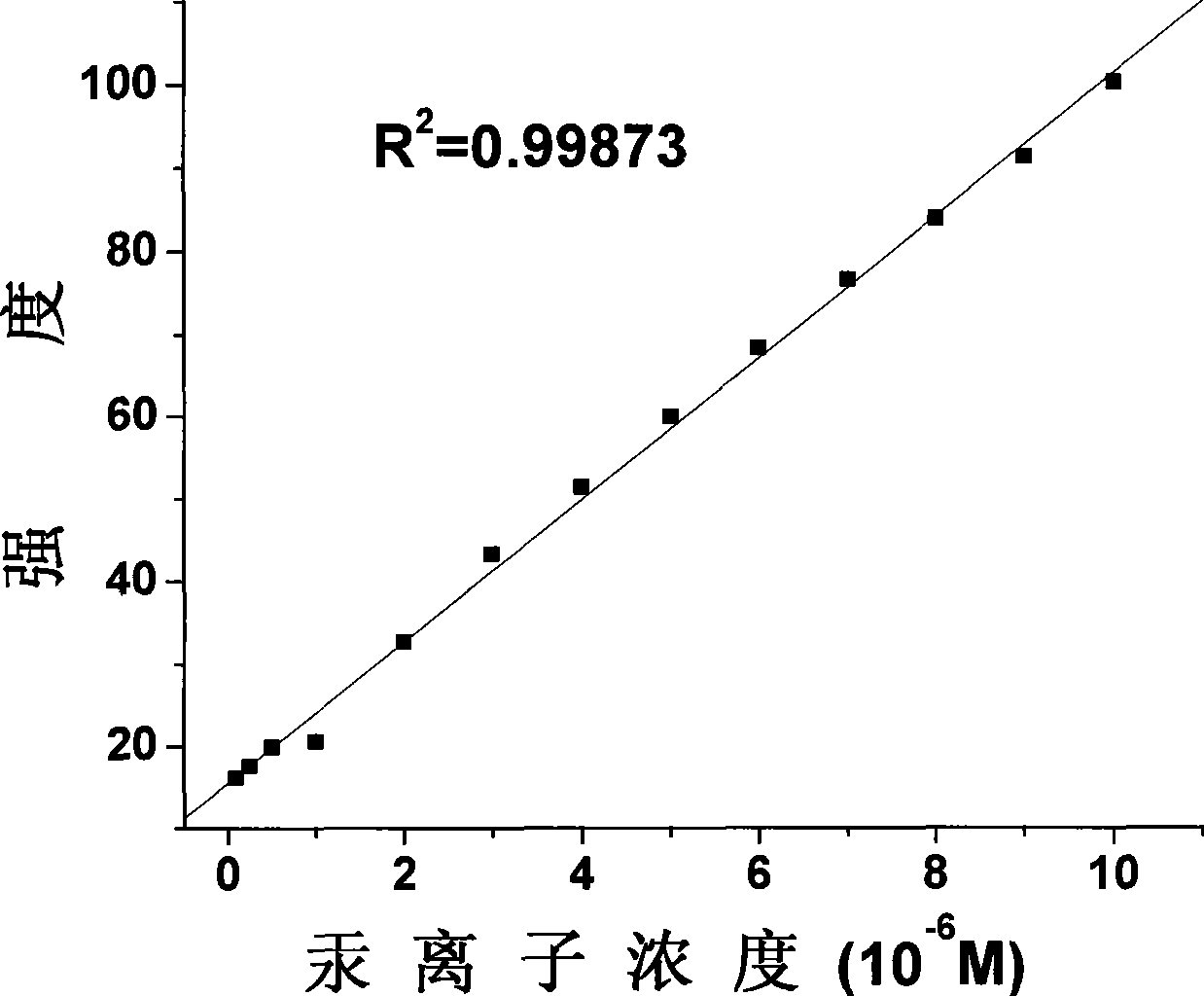
Fluorescent probe for detecting mercury ion in cell, and synthesizing method and usage
A fluorescent probe, mercury ion technology, used in chemical instruments and methods, luminescent materials, biological testing, etc., can solve the problems of poor solubility of the host compound, sensitive to pH changes, long response time, etc., to achieve no toxic side effects, response time Short, easy-to-synthesize effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] (1) Synthesis of rhodamine B acid chloride
[0035] Dissolve 1 g of rhodamine B in 15 mL of dry 1,2-dichloroethane, slowly add 0.5 g of POCl dropwise under stirring 3 , heated to reflux for 4 hours, cooled to room temperature, and removed the solvent under reduced pressure, and the obtained rhodamine B acid chloride crude product was directly used for the next reaction;
[0036] (2) Synthesis of compound (I)-1
[0037] Dissolve 0.5g of 2-mercaptoethylamine hydrochloride in 15mL of dry acetonitrile, add 1.2g of triethylamine, and slowly add 30mL of rhodamine B acid chloride acetonitrile solution dropwise under ice-bath conditions, slowly rise to room temperature and react for 12 hours , the solvent was removed under reduced pressure, and the precipitate was precipitated by adding water. After filtration, the product was vacuum-dried to obtain 0.58 g of the product, with a yield of 50.4%.
[0038] 1 HNMR (CDCl 3 , 400MHz) δ (ppm): 1.12 (12H, t, J = 7.0Hz), 1.59 (1H, s...
Embodiment 2
[0042] (1) Synthesis of rhodamine B acid chloride
[0043] Dissolve 1 g of Rhodamine B in 15 mL of dry 1,2-dichloroethane, slowly add 0.5 mL of POCl dropwise under stirring 3 , heated to reflux for 4 hours, cooled to room temperature, and removed the solvent under reduced pressure, and the obtained rhodamine B acid chloride crude product was directly used for the next reaction;
[0044] (2) Synthesis of compound (II)-1
[0045] Dissolve 0.5g of cystamine dihydrochloride in 15mL of dry acetonitrile, add 1.2mL of triethylamine, and slowly add 30mL of rhodamine B acid chloride in acetonitrile solution dropwise under ice-bath conditions, slowly rise to room temperature and react for 12 hours, The solvent was removed under reduced pressure, and the precipitate was precipitated by adding water. After filtration, the product was vacuum-dried to obtain 0.64 g of the product, with a yield of 55.6%.
[0046] 1 HNMR (CDCl 3 , 400Hz) δ (ppm): 1.17 (24H, t, J = 7.0Hz), 2.27 (4H, q), 3....
Embodiment 3
[0050] (1) Synthesis of rhodamine B acid chloride
[0051] Dissolve 1 g of Rhodamine B in 15 mL of dry 1,2-dichloroethane, slowly add 0.5 mL of POCl dropwise under stirring 3 , heated to reflux for 4 hours, cooled to room temperature, and removed the solvent under reduced pressure, and the obtained rhodamine B acid chloride crude product was directly used for the next reaction;
[0052] (2) Synthesis of compound (II)-1
[0053] Dissolve 0.5g of cystamine dihydrochloride in 15mL of dry acetonitrile, add 1.2mL of triethylamine, and slowly add 30mL of rhodamine B acid chloride in acetonitrile solution dropwise under ice-bath conditions, slowly rise to room temperature and react for 12 hours, The solvent was removed under reduced pressure, and the precipitate was precipitated by adding water. After filtration, the product was vacuum-dried to obtain 0.64 g of the product, with a yield of 55.6%;
[0054] (3) Synthesis of compound (II)-2
[0055] 1.3g of compound (II)-1 and 0.52g ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com