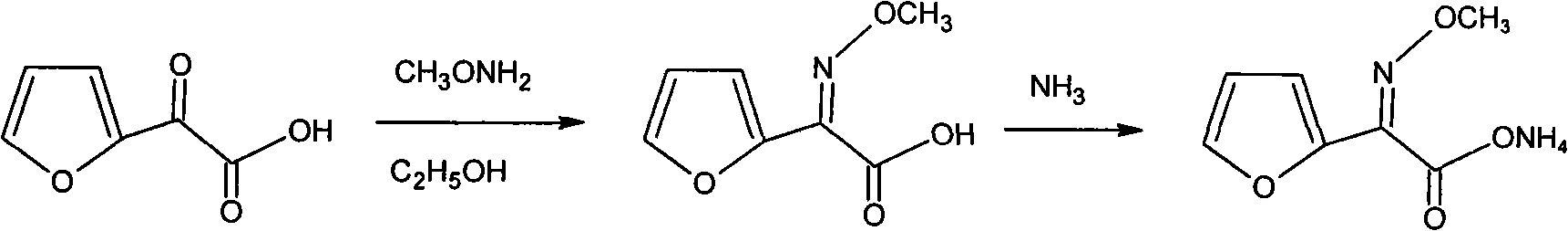
Method for synthesizing (z)-2-(alpha-methoxyimino)furan-ammonium acetate
A technology of ammonium furanoacetate and methoxyimide, applied in the direction of organic chemistry and the like, can solve problems such as unfriendly operating environment and unsatisfactory yield, and achieve the effects of reducing operating links, reducing costs, and improving operating environment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
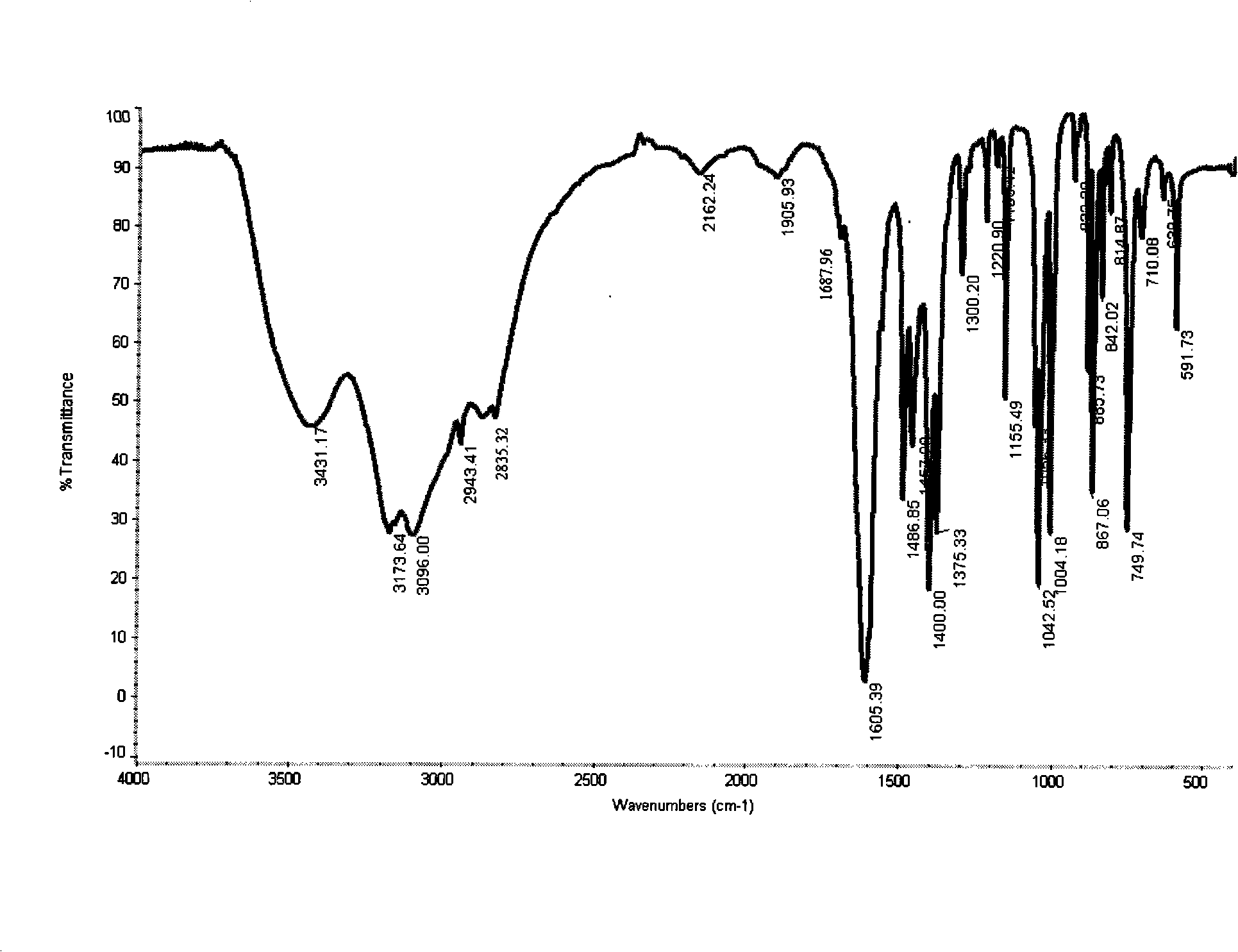
[0031] Add 14.0g (0.1mol) of 99.0% 2-oxo-2-furyl acetic acid, 100mL water, and add 31% hydrochloric acid to adjust the pH to 3.5~4, add 47g of 12% methoxyamine aqueous solution (methoxyamine purity 98%) (0.12mol) at 15~20°C, then keep warm at 20~25°C for 4h, use liquid caustic soda and Hydrochloric acid controls the pH to 3.5-4; after the reaction, adjust the pH of the reaction solution to 0.2 with 31% hydrochloric acid at 15-20°C, then extract it with 100mL chloroform three times, wash the organic layer with saturated brine, dry, and filter; Slowly inject ammonia gas into the organic layer at 5-10°C for about 4 hours until the pH is 7, then keep it warm for 1 hour, and filter to obtain 15.6 g of the crude product; add the crude product to 60 g of methanol, add 2 g of activated carbon, and heat up to reflux for 1 hour , Filtrate while hot to remove activated carbon, then concentrate the mother liquor, distill off about 45g of methanol and cool to 0-5°C, crystallize to give 14....
Embodiment 2
[0034] The methoxyamine aqueous solution is changed into adding methoxyamine hydrochloride 9.2g (0.11mol) in batches, and adds 30% liquid caustic soda of 14.6g, and other is with embodiment 1, the result obtains product 15.1g, yield 81.1%, The liquid phase purity is 99.2%, and the infrared spectrogram is the same as in Example 1.
Embodiment 3
[0036] The amount of water added was changed to 150mL, and the others were the same as in Example 1. As a result, 15.0g of the product was obtained, with a yield of 80.6%, and a liquid phase purity of 99.0%. The infrared spectrogram was the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com