Method for preparing docetaxel
A technology of docetaxel and deacetylation, applied in the field of preparation of docetaxel, can solve the problems of poor reaction selectivity, difficult to obtain high purity, low reaction temperature and the like, and achieves low cost, high reaction yield and high quality. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
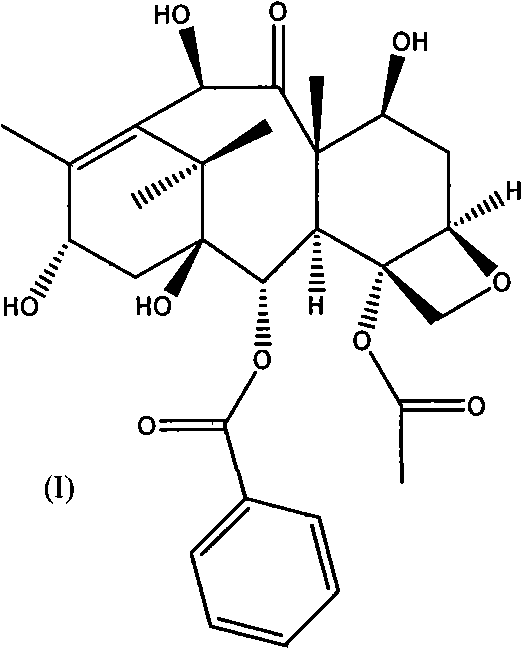
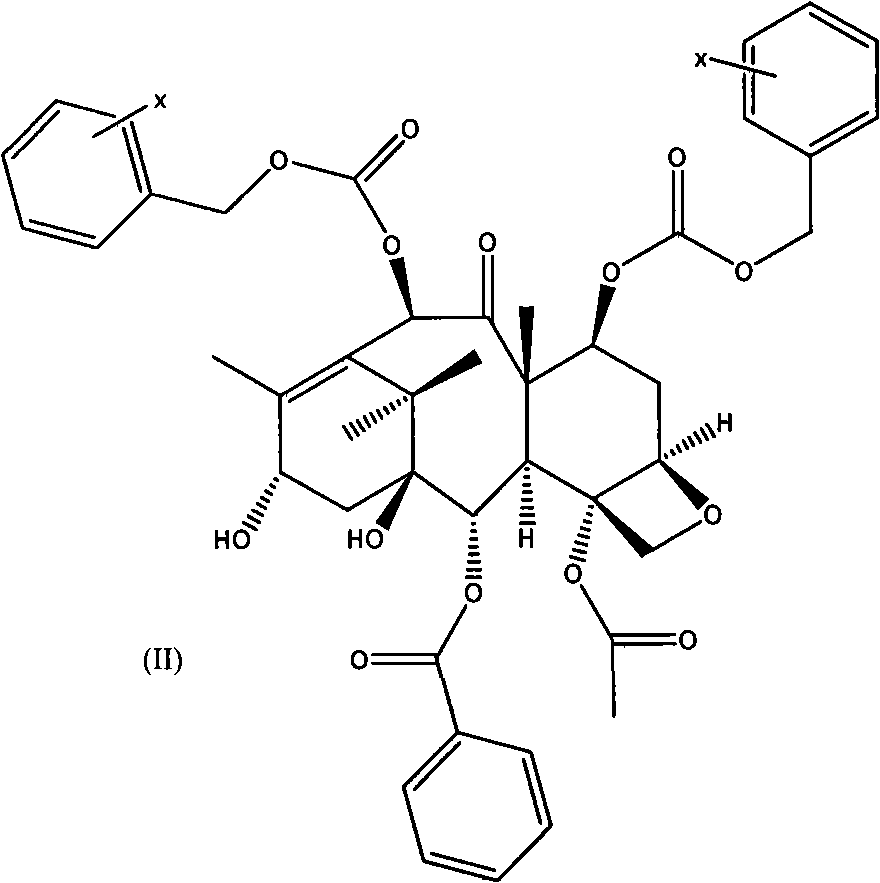
[0061] A. C-7, C-10-di-CBZ-10-deacetylbaccatin III(II)
[0062] The first esterification reaction:
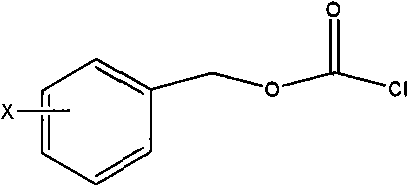
[0063] 10-deacetylbaccatin III (I) and DMAP were dissolved in anhydrous tetrahydrofuran, and the amount of DMAP was 5 times the molar number of 10-deacetylbaccatin III (I); under stirring at room temperature, Benzyl chloroformate (15 times the number of moles of 10-deacetylbaccatin III (I)) was dropped into it, and after the addition was completed, it was reacted at 35-60°C for 0.5 hours, and the reaction solution was cooled to room temperature and then filtered. After adding an appropriate amount of water, adjust the pH of the solution to neutral with a common acid-base, extract with dichloromethane, combine the organic layers, dry with anhydrous sodium sulfate, filter, and evaporate the solvent to obtain C-7, C10-di-X -CBZ-10-deacetylbaccatin III (II) oil crude product; the oil was subjected to silica gel column chromatography (eluent ethyl acetate and n-hexane) to obtain a ...
Embodiment 2
[0074] A.C-7, C-10-di-NO 2 -CBZ-10-Deacetylbaccatin III(II)
[0075] The first esterification reaction:
[0076] Dissolve 10-deacetylbaccatin III (I) and DMAP in anhydrous tetrahydrofuran, the amount of DMAP is 5 times the molar number of 10-deacetylbaccatin III (I); under stirring at room temperature, chlorine Formic acid-4-nitrobenzyl ester (10 times the molar number of 10-deacetylbaccatin III (I)), drop into it, after the addition is completed, react at 35-60°C for 0.5 hours, and the reaction solution is cooled After reaching room temperature, filter, add an appropriate amount of water, adjust the pH of the solution to neutrality with a common acid-base, extract with dichloromethane, combine the organic layers, dry with anhydrous sodium sulfate, filter, and evaporate the solvent to obtain C-7. C10-di-NO 2 -CBZ-10-deacetylbaccatin III (II) oil crude product; the oil was subjected to silica gel column chromatography (eluent ethyl acetate and n-hexane) to obtain a white sol...
Embodiment 3
[0086] A.C-7, C-10-di-Cl-CBZ-10-deacetylbaccatin III(II)
[0087] The first esterification reaction:
[0088] Dissolve 10-deacetylbaccatin III (I) and DMAP in anhydrous tetrahydrofuran, the amount of DMAP is 5 times the molar number of 10-deacetylbaccatin III (I); under stirring at room temperature, chlorine 4-chlorobenzyl formate (10 times the number of moles of 10-deacetylbaccatin III (I)), was added dropwise, and reacted for 0.5 hours at 35-60°C after the addition, and the reaction solution was cooled to After filtering at room temperature, after adding an appropriate amount of water, adjust the pH of the solution to neutrality with common acid and base, extract with dichloromethane, combine the organic layers, dry with anhydrous sodium sulfate, filter, and evaporate the solvent to obtain C-7, C10 -two-no 2 -CBZ-10-deacetylbaccatin III (II) oil crude product; the oil was subjected to silica gel column chromatography (eluent ethyl acetate and n-hexane) to obtain a white so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com