Preparation of Glabridin dispersible tablets and use of the tablets in reducing blood sugar as medicament active composition
A technology of licorice flavonoids and drug activity, which is applied in the field of experimental research on hypoglycemic activity of licorice flavonoids dispersible tablets, can solve the problems of high price, cannot be used for a long time, large toxic and side effects, etc., achieves low cost, is suitable for long-term use, and has toxic and side effects. low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
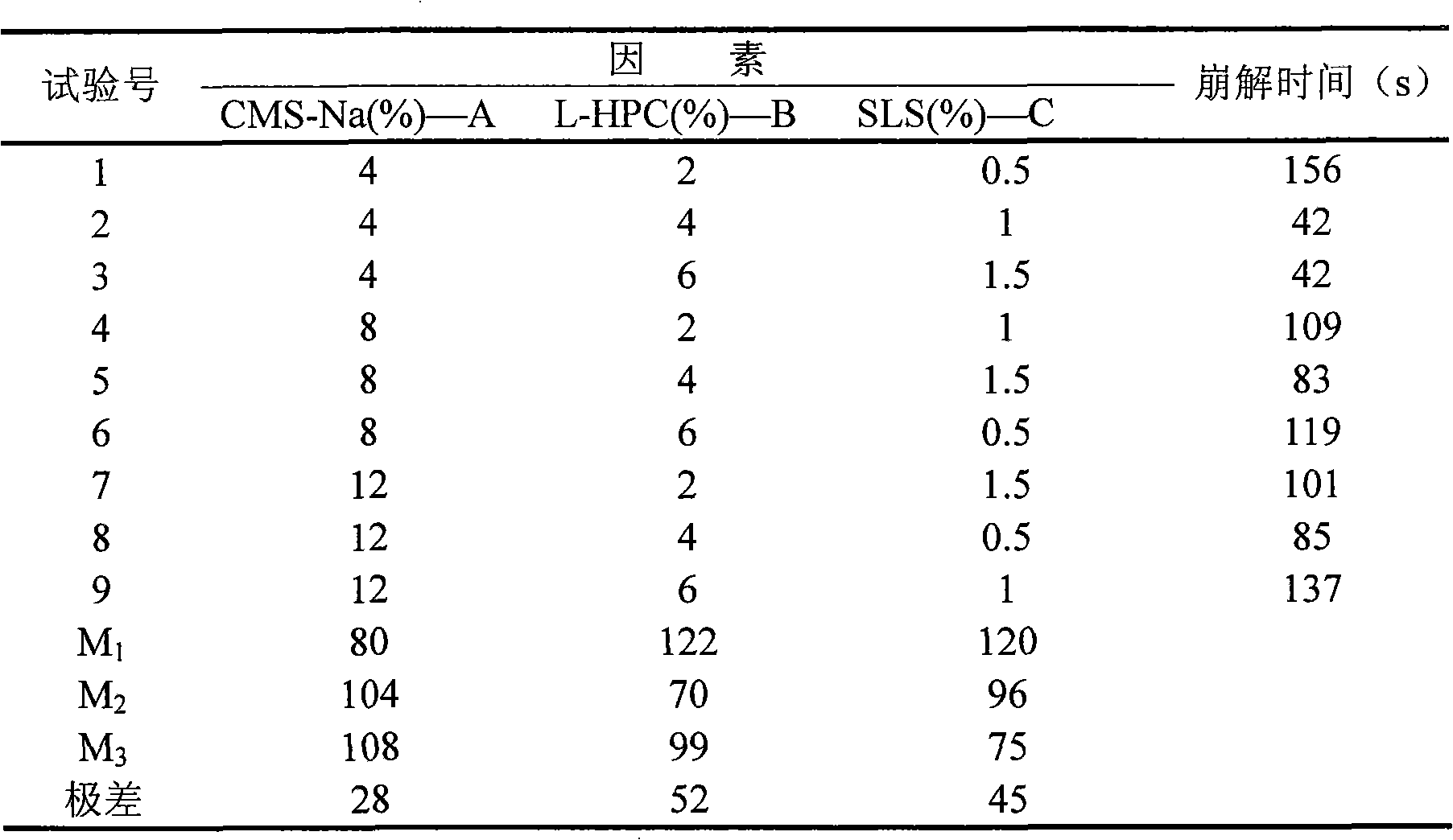
[0019] Weigh 2000g of licorice slices, reflux and extract 3 times according to the ratio of 1:4 of licorice and 95% ethanol, each time for 2h, combine the filtrates 3 times, and recover the solvent by distillation under reduced pressure to obtain 356g of extract, pass the obtained extract through 30-60 mesh Polyamide column chromatography, eluted with water and 95% ethanol in sequence, detected by hydrochloric acid-magnesium powder reaction, combined to obtain total flavonoids. After concentration and freeze-drying, 82.45 g of total flavonoid powder was obtained, with a yield of 4.12%. Weigh 4% CMS-Na, 4% L-HPC, 1.5% SLS and 20% flavonoid powder according to the prescription quantity, add appropriate amount of MCC, pass through a 100 mesh sieve and mix evenly, after drying at 60°C, directly compress into tablets (each tablet contains effective ingredients 250mg).
[0020] Preparation technology of licorice flavone dispersible tablet (LFT)
[0021] (1) optimization of prepara...
experiment example 2
[0050] Experimental Example 2 Application of Licorice Flavone Dispersible Tablets as Active Ingredients in Hypoglycemia
[0051] (1) Experimental study on the effect of licorice flavone dispersible tablet (LFT) on blood sugar in mice
[0052] (1) Effect of Licorice Flavone Dispersible Tablets on Blood Glucose in Alloxan-Induced Diabetic Mice
[0053] Take 85 Kunming mice, half male and half female, and fast for 12 hours. Except for the normal control group, each group is intraperitoneally injected with alloxan (200ml / kg), and the normal control group is intraperitoneally injected with the same amount of normal saline. Glucose level, select the blood glucose value to carry out the test more than 16mmol / L, mice are divided into normal control group, model group, Baitangoping group (25mg / kg), LFT high-dose group (150ml / kg), LFT middle-dose group ( 100mg / kg), the LFT low-dose group (50mg / kg), intragastric administration once a day, the control group and the model group were given...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com